Resuscitation of Acute Decompensated Pulmonary Hypertension: A Prehospital Perspective - JEMS.com
Resuscitation of Acute Decompensated Pulmonary Hypertension: A Prehospital Perspective - JEMS.com |
- Resuscitation of Acute Decompensated Pulmonary Hypertension: A Prehospital Perspective - JEMS.com
- Pulmonary Hypertension With Left-Sided HF: Searching for Appropriate Treatment, Management - The Cardiology Advisor
- 'Bill of Rights' for Cancer Patients: 10 Principles for Care - Medscape
- Pulmonary tumour thrombotic microangiopathy - CMAJ
| Resuscitation of Acute Decompensated Pulmonary Hypertension: A Prehospital Perspective - JEMS.com Posted: 10 Mar 2020 08:33 AM PDT   IntroductionPulmonary hypertension (PH) is a chronic disease associated with significant morbidity and mortality, responsible for approximately 15,000 deaths per year in United States.1 The deleterious cardiac effects associated with PH (e.g. Right Ventricular disease) render this population particularly susceptible to decompensation. Hypoxemia, acidosis and hypercapnia are not well tolerated in PH and will acutely increase pulmonary artery pressures, leading to hemodynamic collapse.2 This is especially problematic as this instability is further exacerbated by standard resuscitation practices. Despite an evident increase in research regarding the resuscitation of acute decompensated PH, many emergency medical services (EMS) protocols, paramedic education programs, emergency medicine residencies and critical care fellowships still fail to highlight the clinical importance of PH in resuscitation — subjecting this patient population to significant harm.2 The purpose of this review is to provide an overview of PH, outline the relevance of PH to EMS and help guide the development of future EMS protocols and education programs. DefinitionPH is definitively diagnosed by right-sided heart catheterization to assess mean pulmonary artery pressure (mPAP). Normal mean pulmonary artery pressure (mPAP) is defined as 14 +/- 3 mmHg, with PH being defined as a mPAP > 25 mmHg.3 PH is then subdivided into five groups based on specific pathogenesis, each with unique treatment trajectories. Group One PH is a result of pathologies causing direct harm to the pulmonary arteries and is frequently referred to as pulmonary arterial hypertension (PAH). PAH is further divided into the following subgroups: heritable pulmonary hypertension, drug induced PAH, PAH associated with connective tissue disorders, portal hypertension, HIV infection, Schistosomiasis, congenital heart defects and idiopathic cases of PH. Group Two PH is a result of left sided heart disease, while Group Three is PH second to obstructive lung diseases such as Chronic Obstructive Pulmonary Disease (COPD). Together, Groups Two and Three make up the majority of PH cases.4 Group Four PH arises from coagulation issues and pulmonary artery obstructions and is the only potentially reversible form of PH. Lastly, Group Five PH refers to cases that do not fit any of the previous categories. Group Five cases are often complex and multifactorial and are split into four broad categories including: hematologic disorders, systemic disorders, metabolic disorders and other disorders.3 Identifying the specific subtype of PH is not reasonable in the prehospital setting; however, a general understanding of the breadth of possible etiologies and subsequent inquiry by prehospital providers may help guide treatment strategies in pre-hospital shock resuscitation. Pathophysiology of Pulmonary HypertensionPulmonary hypertension (PH) is a progressive disease. Prognosis is directly related to the speed of disease progression and the ability for the body to compensate against increased pulmonary vascular resistance (PVR).5 Increased PVR is the downstream result of the distinct underlaying condition that lead to PH. Over time, the right ventricle undergoes hypertrophy to maintain cardiac output (CO) in the face of elevated PVR.6 However, chronic exposure to increased PVR and elevated right ventricular pressures eventually results in dilation of the right ventricle through backflow from the pulmonary vasculature across an incompetent pulmonic valve. Dilation of the right ventricle is indicative of end-stage PH and is associated with poor prognosis and significant risk of hemodynamic collapse.7 Hemodynamic compromise in this stage results from right ventricular failure and subsequent reductions of CO below homeostatic levels. This may occur as part of the natural progression of the disease or may be precipitated by a number of external triggers such as noncompliance with medication, excessive salt intake, infection, or acute thromboembolic events (e.g. pulmonary embolism).8 Mechanisms Behind Right Ventricular Failure and Hemodynamic CollapseAcute exacerbations of PH from either natural disease progression or external triggers require specific interventions that necessitate a foundational understanding of the pathophysiology leading to right ventricular failure and hemodynamic collapse. These treatment strategies may significantly differ from the care of similar conditions in patients without PH — particularly in the management of septic shock.9 Hemodynamic compromise occurs when the right ventricle can no longer compensate against increased PVR and subsequent dilation of the RV occurs second to backflow from the pulmonary vasculature. In a normal heart, expanded volume yields an inotropic effect and increases stroke volume (SV) via the Frank-Starling Mechanism. However, once the right ventricle is expanded beyond a critical point, SV will decrease worsening backflow and ventricular dilation. The over-expanded right ventricle causes the interventricular septum to bulge into the left ventricle (LV), resulting in decreased left ventricular SV and reduced cardiac output (CO) in a phenomenon called interventricular dependence.10 At the same time, tricuspid valve regurgitation increases right atrial pressure, leading to further reduction of LV preload and subsequent impedance of CO.11 Adding to the insult on compensatory mechanisms, high atrial pressures at this point may render a formerly asymptomatic foramen ovale to become patent and allow interatrial mixing, affecting blood oxygenation.12 Additionally, this physiology has adverse effects on the perfusion of the right coronary artery (RCA). Under standard conditions, blood flow to the RCA relies on a pressure gradient between the aorta and the RV, during systole and diastole. As RV pressures approach systemic pressures in acute decompensated PH, there is substantial risk of RV ischemia through several mechanisms out of the scope of this review. This resulting mismatch between oxygen supply and demand reduces RV contractility, leading to further reductions in CO.13–15 The previous cascade produces diastolic and systolic right heart failure, which can lead to multi-organ failure. Specifically, renal failure arises through sympathetic activation of the renin-angiotensin-aldosterone system and endogenous vasopressin release, which together impair perfusion through fluid retention and arterial vasoconstriction.5 Overall, this volume overloaded state with associated hemodynamic collapse requires a complex and unique approach to prehospital shock resuscitation. Identification in the Prehospital EnvironmentIdentification of PH in the prehospital arena is difficult and relies heavily on past medical history. In the absence of a self-reported reported diagnosis, several factors may help identify patients with PH. Prescribed calcium channel blockers, endothelin receptor antagonists, phosphodiesterase-5 (PDE5) inhibitors and prostanoids are all common in the treatment of PH (See Table 1).4 However, these medications are not solely used for PH and subsequently do not definitively indicate a past diagnosis of PH. Additionally, patients with a history of COPD, left-sided heart failure, connective tissue disorders, thromboembolic disease, or low socioeconomic status have a higher prevalence of undiagnosed PH.4 Common electrocardiogram findings include right axis deviation, right ventricular hypertrophy, right bundle branch block or T-wave inversion, although ECG lacks the sensitivity and precision to possess diagnostic value.16,17 As a result of non-descriptive physical exam findings, it is imperative for prehospital providers to obtain a full medical history from the patient or family when making decisions regarding resuscitation management. In the emergency department, ultrasound has been increasingly utilized in the identification of various pathologies and is positioned to continue to expand its role in the near future.18 Although definitive diagnosis of PH requires right heart catheterization, ultrasound may play a role in the emergent setting.2,16 The apical four chamber and parasternal short axis views identify the presence of RV dilation and septal bulging respectively and have been successfully used to guide resuscitation in the emergency department.19–22 Formal echocardiography, including tissue doppler, may be superior, but is not realistic in the emergency department or prehospital setting. Furthermore, ultrasound in the prehospital setting is currently limited to simple yes/no diagnoses such as the presence or absence of cardiac activity.23 As a result, the recognition of RV dilation and septal bulging are likely unrealistic and would require extensive training. An alternative to rigorous training is the use of tele-ultrasound (TUS), which allows paramedics to perform ultrasound under the guidance of an emergency physician, who will ultimately interpret the findings.24,25 The ability of emergency physicians to guide and interpret this form of ultrasonography currently varies by institution; however, this skillset may become universal as the role of ultrasound in emergency medicine continues to grow.26 Fluid ResuscitationThe resuscitation of patients with PH presenting in shock is particularly complex. In the setting of septic shock, patients may be subject to harm if treated with traditional sepsis protocols.2 In general, guidelines suggest an initial crystalloid fluid bolus of approximately 30cc/kg or 1-2 liters.27 In a normal patient, this fluid increases perfusion through raising both CO and mean arterial pressure (MAP). However, in the setting of PH, large fluid boluses will exacerbate dilation of an already volume overloaded RV, leading to greater interventricular dependence and a subsequent reduction in CO.2 Additionally, RV dilation due to volume-overload will raise RV pressures. As RV pressure approaches systemic blood pressure — which is likely low in the setting of shock — RCA perfusion will decrease as a result of reliance on diastolic filling.3 Likewise, in other shock etiologies, large fluid boluses will have similar detrimental effects. Therefore, prehospital shock resuscitation in the setting of PH should cautiously employ fluids, limited to small 250cc boluses, only when there are clear indications of volume depletion.2 VasopressorsThe use of vasopressors in the management of acute decompensated PH is complicated and guided by a sound understanding of physiology. Patient's in shock second to decompensated PH will present with increased PVR, decreased SVR and poor myocardial contractility — all resulting in reductions in CO. As systemic hypotension is extremely detrimental in these patients due to the reduction of RCA perfusion described above, vasopressors must be employed early in the course of care (See Table 2). Push-dose EpinephrineIn many states, EMS is not permitted to administer vasopressors via intravenous infusion in the field and is limited to the use of Push-Dose-Epinephrine (PDE). Traditionally, PDE is used as a temporizing agent to raise blood pressure in peri-arrest patients who are exhibiting signs of shock and hemodynamic collapse. In PH, the threshold to use PDE may be lower as PDE could be a suitable answer to increasing systemic blood pressure above RV pressures in an effort to maintain RCA perfusion; however, this has not been studied to our knowledge and should be employed cautiously.28 Additionally, epinephrine has important considerations for use in patients with PH. With alpha and beta effects, epinephrine will increase CO and SVR, but comes with an increased risk of tachydysrhythmias and increased PVR.29,30 Tachydysrhythmias are particularly harmful in this population as they reduce ventricular filling times, thus reducing CO.29,30 As a result, tachydysrhythmias encountered in the setting of PH should be promptly addressed. Several studies have shown that conversion to sinus rhythm, not rate control alone, is important in the cohort.31 With the determinantal effects of systemic hypotension in these patients, electrical cardioversion may be preferred to chemical cardioversion. NorepinephrineIn EMS agencies that allow for intravenous infusion of vasopressors, norepinephrine is generally accepted as the first line treatment when distributive shock is suspected.27,32 As a strong alpha one agonist, norepinephrine will help offset hypotension by increasing SVR. At the same time, norepinephrine may increase PVR, a potentially harmful side effect in PH. Norepinephrine, like epinephrine, has some beta-1 effects, but they are not as profound and have not been associated with an increased risk of tachydysrhythmias.29 VasopressinRecent evidence has suggested that vasopressin may be a first line agent for resuscitation of decompensated PH. Low-dose vasopressin has been found to increase SVR through V1 stimulation, but unlike norepinephrine, it also provides a reduction in PVR.33 The combination of increased SVR with decreased PVR makes vasopressin a potential first line agent in PH resuscitation, although further research is required. PhenylephrineAs an alpha-1 agonist, phenylephrine has non-selective vasoconstrictive effects on both systemic and pulmonary vasculature, resulting in increases in both SVR and PVR without an effect on heart rate or contractility.34 The associated increase in PVR with the administration phenylephrine is potentially deleterious in this patient population as it will further increase RV workload, leading to reductions in CO. DopamineDopamine is the biological precursor to norepinephrine and its effects are highly dependent on dosing. At low doses (3-5mcg/kg/min), dopamine produces systemic vasodilation and increased blood flow to the renal tubules, resulting in sodium excretion and increased urine output. At moderate doses (3-10 mcg/kg/min), dopamine has positive inotropic and chronotropic effects through beta receptor stimulation. High doses (>10 mcg/kg/min), produces systemic and pulmonary vasoconstriction through alpha-1 stimulation. The pulmonary vasoconstriction may increase PVR and worsen RV failure; however, studies examining this theory are mixed.35–37 Additionally, tachydysrhythmias are reported in about 25% of patient receiving dopamine, which is especially harmful in PH as described previously.38 DobutamineDue to reduced RCA perfusion and rising PVR, inotropes may be required. The most commonly used inotropes in this setting are epinephrine, dobutamine and milrinone. Dobutamine belongs to a class of drugs referred to as inodilators, meaning it has agonistic effects on both beta one and two receptors, leading to an increase in heart rate and contractility and a decrease in SVR. As a result of the potential risk for hypotension, dobutamine should only be employed in this setting if a vasopressor is running to offset the decrease in SVR.29 MilrinoneLike dobutamine, milrinone is also an inodilator. However, milrinone produces an increase in contractility and a decrease in SVR through phosphodiesterase inhibition, not beta stimulation. In theory, this should carry less risk of tachydysrhythmias, a concern for the use of dobutamine in this setting.29 Additionally, nebulized milrinone may be of use to achieve a reduction in PVR without the associated risk of hypotension.39 However, this route of administration will not increase myocardial contractility to the same degree. EpinephrineEpinephrine can also be employed to produce positive inotropic effects. Epinephrine effects both beta 1 and 2 receptors, although as an alpha-1 agonist, it offsets the beta-2 reduction in SVR and typically yields a net increase in SVR.30 As described above, epinephrine is potentially harmful in the population due to its risks of tachydysrhythmias and increased PVR. Overall, in a perfect world there would be a single agent that would achieve a reduction in PVR associated with an increase in SVR and myocardial contractility. To our knowledge this agent does not exist and hemodynamic management must rely upon a complex combination of a variety of vasoactive medications. Therefore, prompt transport to a tertiary center with PH specialists is extremely important. Airway ManagementOxygenation in patients with PH is especially important. Even transient hypoxia in this cohort can raise PVR and accelerate the cascade of events leading to hemodynamic collapse through a mechanism called hypoxic pulmonary vasoconstriction (HPV). HPV is generally a beneficial mechanism that shunts blood to only well-ventilated portions of the lung, optimizing oxygenation. This is especially important in compensation of chronic lung diseases such as COPD. However, HPV also results in an increase in pulmonary artery pressure, presenting a risk to patients with PH.40 In order to combat this process, high flow oxygen via non-rebreather mask should be promptly initiated and may be supplemented with an additional high flow nasal cannula.41 Patients should also be placed in a semi-fowlers position to avoid increased PVR associated with supine positioning.42 If oxygen saturations still do not climb with use of the non-rebreather and nasal cannula, Continuous-Positive Pressure Ventilation (CPAP) should be employed with the lowest possible Peak-End-Expiratory-Pressure (PEEP), generally 5 cm H2O.43 Higher PEEP may increase pulmonary artery pressures.43–45 If these methods still do not raise oxygen saturation, intubation should be considered. Typically, early intubation in patients presenting with severe shock is recommended in efforts to better control a patient's ventilation, oxygenation, hemodynamics and airway protection. In the case of PH, this may approach may be detrimental.2,16,43 In fact, the use of mechanical ventilation in PH has been previously associated with a 26.5% increase in mortality.46 Patients with PH are particularly susceptible to the effects of positive pressure ventilation (PPV) as PPV causes an increase in intrathoracic pressure, resulting in higher pulmonary artery and RV pressures. PPV also contributes to hyperinflation of the alveoli which compress pulmonary vessels, raising RV afterload.16 Additionally, induction agents used in rapid sequence intubation (RSI) are often associated with hypotension and poor cardiac function which as described previously reduces RCA perfusion.47,48 RSI may also cause hypoxia and hypercapnia during laryngoscopy, which is especially harmful in this patient population.49 To avoid hypoxia, pre-oxygenation with a high flow nasal cannula (15 lpm) should be employed. CPAP may also be of benefit in the pre-oxygenation of these patients as it provides a low level of positive pressure which will minimize the shock experienced by the body when PPV is initiated after intubation.42 If intubation is to be performed it should be after exhausting other methods of oxygenation and be performed by the most experienced intubator with careful attention paid to agents selected for induction. Etomidate and ketamine are generally preferred over propofol as the risk of hypotension is significantly less.42 Additionally, some evidence suggests an awake approach to intubation may be preferred in patients at risk of hemodynamic collapse.42 Once a patient is intubated ventilator settings should follow the lung protective strategy with several caveats.50 Hypercapnia is generally well accepted in most patients, but is harmful due to exacerbations of HPV in this setting.51 Therefore, the respiratory rate should be set higher than typical guidelines at around 16 bpm as opposed to 10-12 bpm. Additionally, minimal PEEP (<12 cm H2O) should be used in an effort to avoid an increase in pulmonary artery pressure.1 In summary, airway management in PH differs significantly from typical resuscitation and is particularly sensitive to transient hypoxia and hypercapnia. As a result, close attention should be paid to the oxygenation and ventilatory status of these patients. ConclusionResuscitation of patients with acute decompensated PH is complex and often does not receive adequate attention in the prehospital field. In spite of this, it is important that PH is recognized prehospitally as resuscitation by standard protocols will cause harm to this population before they ever arrive at the emergency department doors. EMS agencies, institutions and providers should increase awareness of the special considerations for resuscitation in this population as well as conduct future research to understand the best approach to the management of this condition in the prehospital environment. References1. Hyduk A, Croft J, Ayala C, Zheng K, Zheng Z-J, Mensah G. Pulmonary Hypertension Surveillance — United States, 1980–2002. MMWR. 2005 Nov 11;54(5):1–28. 2. Wilcox SR, Kabrhel C, Channick RN. Pulmonary Hypertension and Right Ventricular Failure in Emergency Medicine. Annals of Emergency Medicine. 2015 Dec;66(6):619–28. 3. Alves-Jr J, Oleas F, Souza R. Pulmonary Hypertension: Definition, Classification, and Diagnosis. Semin Respir Crit Care Med. 2017 Oct;38(05):561–70. 4. Wijeratne DT, Lajkosz K, Brogly SB, Lougheed MD, Jiang L, Housin A, et al. Increasing Incidence and Prevalence of World Health Organization Groups 1 to 4 Pulmonary Hypertension: A Population-Based Cohort Study in Ontario, Canada. Circ Cardiovasc Qual Outcomes [Internet]. 2018 Feb [cited 2020 Jan 14];11(2). Available from: https://www.ahajournals.org/doi/10.1161/CIRCOUTCOMES.117.003973. 5. Savale L, Weatherald J, Jaïs X, Vuillard C, Boucly A, Jevnikar M, et al. Acute decompensated pulmonary hypertension. Eur Respir Rev. 2017 Dec 31;26(146):170092. 6. Kret M, Arora R. Pathophysiological Basis of Right Ventricular Remodeling. J Cardiovasc Pharmacol Ther. 2007 Mar;12(1):5–14. 7. McLaughlin VV, Gaine SP, Howard LS, Leuchte HH, Mathier MA, Mehta S, et al. Treatment Goals of Pulmonary Hypertension. Journal of the American College of Cardiology. 2013 Dec;62(25):D73–81. 8. Sztrymf B, Souza R, Bertoletti L, Jaïs X, Sitbon O, Price LC, et al. Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. European Respiratory Journal. 2010 Jun 1;35(6):1286–93. 9. Price LC, Dimopoulos K, Marino P, Alonso-Gonzalez R, McCabe C, Kemnpy A, et al. The CRASH report: emergency management dilemmas facing acute physicians in patients with pulmonary arterial hypertension. Thorax. 2017 Nov;72(11):1035–45. 10. Haddad F, Guihaire J, Skhiri M, Denault AY, Mercier O, Al‐Halabi S, et al. Septal Curvature Is Marker of Hemodynamic, Anatomical, and Electromechanical Ventricular Interdependence in Patients with Pulmonary Arterial Hypertension. Echocardiography. 2014;31(6):699–707. 11. Maeder MT, Holst DP, Kaye DM. Tricuspid Regurgitation Contributes to Renal Dysfunction in Patients With Heart Failure. Journal of Cardiac Failure. 2008 Dec;14(10):824–30. 12. Sharan L, Stackhouse K, Awerbach JD, Bashore TM, Krasuski RA. Effect of Patent Foramen Ovale in Patients With Pulmonary Hypertension. The American Journal of Cardiology. 2018 Aug;122(3):505–10. 13. Ryan JJ, Archer SL. The Right Ventricle in Pulmonary Arterial Hypertension: Disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res. 2014 Jun 20;115(1):176–88. 14. Matthews J, McLaughlin V. Acute Right Ventricular Failure in the Setting of Acute Pulmonary Embolism or Chronic Pulmonary Hypertension: A Detailed Review of the Pathophysiology, Diagnosis, and Management. CCR. 2008 Feb 1;4(1):49–59. 15. Mebazaa A, Karpati P, Renaud E, Algotsson L. Acute right ventricular failure—from pathophysiology to new treatments. Intensive Care Med. 2004 Feb;30(2):185–96. 16. Humbert M, Vachiery J-L, Barbera JA, Beghetti M, Corris P, Gaine S, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). European Heart Journal. 2009 Oct 2;30(20):2493–537. 17. Nelson B, Sanghvi A. Out of hospital point of care ultrasound: current use models and future directions. Eur J Trauma Emerg Surg. 2016;2016(42):139–50. 18. Ultrasound Guidelines: Emergency, Point-of-Care and Clinical Ultrasound Guidelines in Medicine. Annals of Emergency Medicine. 2017 May;69(5):e27–54. 19. Andrus P, Dean A. Focused Cardiac Ultrasound. Global Heart. 2013 Dec;8(4):299–303. 20. Dela Cruz M, Devey JS. Emergency department diagnosis of pulmonary hypertension in a patient with left atrial sarcoma. Int J Emerg Med. 2014 Dec;7(1):32. 21. Dessie A, Leung S, D'Amico B, Fischer KA, Binder Z, Abo A. Focused cardiac ultrasound to expedite diagnosis of pulmonary hypertension in children in the emergency department: A case series. The American Journal of Emergency Medicine. 2019 Nov;S0735675719307545. 22. Olaes K, Mailhot T, Perera P. Pulmonary Hypertension, Hemoptysis and an Echocardiographic Finding of a Ventricular Septal Defect. WestJEM. 2012 Dec 1;13(6):516. 23. Bøtker MT, Jacobsen L, Rudolph SS, Knudsen L. The role of point of care ultrasound in prehospital critical care: a systematic review. Scand J Trauma Resusc Emerg Med [Internet]. 2018 Jun 26; 26. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6019293/. 24. Boniface KS, Shokoohi H, Smith ER, Scantlebury K. Tele-ultrasound and paramedics: real-time remote physician guidance of the Focused Assessment With Sonography for Trauma examination. The American Journal of Emergency Medicine. 2011 Jun;29(5):477–81. 25. Eder P, Reime B, Wurmb T, Kippnich U, Shammas L, Rashid A. Prehospital Telemedical Emergency Management of Severely Injured Trauma Patients: A Systematic Review. Methods Inf Med. 2018 Nov;57(05/06):231–42. 26. Lewiss RE, Pearl M, Nomura JT, Baty G, Bengiamin R, Duprey K, et al. CORD-AEUS: Consensus Document for the Emergency Ultrasound Milestone Project. Burton J, editor. Acad Emerg Med. 2013 Jul;20(7):740–5. 27. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017 Mar;43(3):304–77. 28. Nawrocki PS, Poremba M, Lawner BJ. Push Dose Epinephrine Use in the Management of Hypotension During Critical Care Transport. Prehospital Emergency Care. 2019 Feb 26;1–8. 29. Hollenberg SM. Vasoactive Drugs in Circulatory Shock. Am J Respir Crit Care Med. 2011 Apr;183(7):847–55. 30. Myburgh JA, Higgins A, Jovanovska A, Lipman J, Ramakrishnan N, Santamaria J, et al. A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med. 2008 Dec;34(12):2226–34. 31. Tongers J, Schwerdtfeger B, Klein G, Kempf T, Schaefer A, Knapp J-M, et al. Incidence and clinical relevance of supraventricular tachyarrhythmias in pulmonary hypertension. American Heart Journal. 2007 Jan;153(1):127–32. 32. Melzer D. Dopamine versus Norepinephrine in the Treatment of Septic Shock: A Meta-analysis. The Journal of Emergency Medicine. 2012 Jun;42(6):751. 33. Indrambarya T, Boyd JH, Wang Y, McConechy M, Walley KR. Low-dose vasopressin infusion results in increased mortality and cardiac dysfunction following ischemia-reperfusion injury in mice. Crit Care. 2009;13(3):R98. 34. Rich S, Gubin S, Hart K. The Effects of Phenylephrine on Right Ventricular Performance in Patients with Pulmonary Hypertension. Chest. 1990 Nov;98(5):1102–6. 35. Holloway EL, Polumbo RA, Harrison DC. Acute circulatory effects of dopamine in patients with pulmonary hypertension. Br Heart J. 1975 May;37(5):482–5. 36. Leier C V, Heban P T, Huss P, Bush C A, Lewis R P. Comparative systemic and regional hemodynamic effects of dopamine and dobutamine in patients with cardiomyopathic heart failure. Circulation. 1978 Sep 1;58(3):466–75. 37. Peter Steen, Tinker J, Pluth J, Barnhorst D, Tarhan S. Efficacy of Dopamine, Dobutamine and Epinephrine During Emergence from Cardiopulmonary Bypass in Man. Circulation. 1977 Sep 6;57(2):378–84. 38. Daniel DB, Patrick B, Jacques D, Christian M, Didier C, Cesar A, et al. Comparison of Dopamine and Norepinephrine in the Treatment of Shock. n engl j med. 2010;11. 39. Buckley MS, Feldman JP. Nebulized Milrinone Use in a Pulmonary Hypertensive Crisis. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2007;27(12):1763–6. 40. Michelakis E. Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. Journal of Molecular and Cellular Cardiology [Internet]. 2004 Nov [cited 2020 Jan 17]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022282804002780. 41. Weingart SD, Levitan RM. Preoxygenation and Prevention of Desaturation During Emergency Airway Management. Annals of Emergency Medicine. 2012 Mar;59(3):165-175.e1. 42. Johannes J, Berlin DA, Patel P, Schenck EJ, West FM, Saggar R, et al. A Technique of Awake Bronchoscopic Endotracheal Intubation for Respiratory Failure in Patients With Right Heart Failure and Pulmonary Hypertension: Critical Care Medicine. 2017 Sep;45(9):e980–4. 43. Hoeper MM, Granton J. Intensive Care Unit Management of Patients with Severe Pulmonary Hypertension and Right Heart Failure. Am J Respir Crit Care Med. 2011 Nov 15;184(10):1114–24. 44. Vargas M, Sutherasan Y, Gregoretti C, Pelosi P. PEEP Role in ICU and Operating Room: From Pathophysiology to Clinical Practice. The Scientific World Journal. 2014;2014:1–8. 45. Venkataraman ST, Allen JW. PEEP-induced pulmonary vasoconstriction in neonatal piglets: Analysis by pressure-flow curves. Pediatric Pulmonology. 1999;28(1):12–7. 46. Rush B, Biagioni BJ, Berger L, McDermid R. Mechanical Ventilation Outcomes in Patients With Pulmonary Hypertension in the United States: A National Retrospective Cohort Analysis. J Intensive Care Med. 2017 Dec;32(10):588–92. 47. Gordon C, Collard CD, Pan W. Intraoperative management of pulmonary hypertension and associated right heart failure: Current Opinion in Anaesthesiology. 2010 Feb;23(1):49–56. 48. Höhn L, Schweizer A, Morel DR, Spiliopoulos A, Licker M. Circulatory Failure after Anesthesia Induction in a Patient with Severe Primary Pulmonary Hypertension: Anesthesiology. 1999 Dec;91(6):1943. 49. Dalabih M, Rischard F, Mosier JM. What's new: the management of acute right ventricular decompensation of chronic pulmonary hypertension. Intensive Care Med. 2014 Dec;40(12):1930–3. 50. Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med. 2017 Feb 15;195(4):438–42. 51. Paternot A, Repessé X, Vieillard-Baron A. Rationale and Description of Right Ventricle-Protective Ventilation in ARDS. Respiratory Care. 2016 Oct 1;61(10):1391–6. |
| Posted: 24 Feb 2020 12:00 AM PST  Pulmonary hypertension (PH) can be a severe and potentially debilitating complication in patients with chronic left-sided heart failure (HF). In a review published in Heart Failure Clinics, researchers from Germany and Italy provided recommendations on assessment and an overview of targeted management strategies that may be helpful for these patients. The presence of PH in patients with left-sided HF can confer poor prognosis and survival, making appropriate assessment of the condition crucial for optimizing outcomes. According to the researchers, right heart catheterization or a fluid challenge may be indicated in some patients at intermediate risk in an effort to obtain a correct diagnosis. A pulmonary arterial wedge pressure (PAWP) of ≥15 mm Hg is a mandatory criterion for establishing PH associated with left-sided HF. Guidelines recommend that PAWP should be assessed at end-expiration at rest using a proper "zero" point at the mid-chest. A provocative test should be used to confirm PAWP findings in patients with borderline PAWP values (ie, 13-15 mm Hg) or in patients with lower PAWP but who present with echocardiographic signs of left ventricular hypertrophy. There are currently no approved targeted therapies for patients with PH and right HF with preserved ejection fraction (EF) or HF with reduced EF. Clinical trials are currently ongoing to test drugs that either activate guanylate cyclase or inhibit phosphodiesterase type 5 in patients with PH and HF with preserved EF. These studies include the DYNAMIC trial (Pharmacodynamic Effects of Riociguat in Pulmonary Hypertension and Heart Failure With Ejection Fraction; ClinicalTrials.gov Identifier: NCT02744339) and the PASSION Study (Phosphodiesterase-5 Inhibition in Patients With HF With Preserved Ejection Fraction and Combined Post- and Pre-Capillary PH; European Union Clinical Trials Register No.: 2017-003688-37). The investigators suggested exercise training, a guideline-recommended treatment, may be beneficial in some individuals. In the context of PH with left-sided HF, exercise training may be associated with improvements in exercise capacity. In patients with right ventricular failure whose prognoses do not improve despite use of vasopressors and inotropes, mechanical circulatory is often necessary. "A profound understanding of the mechanisms that may lead to the development of PH [caused by chronic left-sided HF] might be helpful to improve the management of these patients," the researchers wrote. Reference Marra A-M, Benjamin N, Cittadini A, Bossone E, Grünig E. When pulmonary hypertension complicates heart failure. Heart Fail Clin. 2020;16(1):53-60. This article originally appeared on Pulmonology Advisor |
| 'Bill of Rights' for Cancer Patients: 10 Principles for Care - Medscape Posted: 11 Mar 2020 08:11 AM PDT  In spite of oncologists' best efforts, the divide between what they provide and what patients experience continues to limit the delivery of high-quality, patient-centered cancer care in the United States, says a group of experts. Good intentions are not enough to close the gap, say Joseph O. Jacobson, MD, of the Dana Farber Cancer Institute and Harvard Medical School, Boston, Massachusetts, and colleagues. "Oncologists naturally believe that the care they deliver is patient focused, a core part of their mission," they write. "Nevertheless, many patients with cancer perceive gaps in their care experience," they continue. Patients report concerns such as "excessive waiting for appointments, for scheduled interventions to begin, and for return phone calls; a lack of coordination among their multiple clinicians; unclear answers about disease prognosis; and daunting financial challenges, frequently with little pertinent assistance." The complete systems overhaul that is needed to shift the current state of cancer care is too big a job for oncologists to tackle on their own, they emphasize in an editorial published February 10 in JCO Clinical Practice. To get the ball rolling, Jacobson and colleagues propose a bill of rights for patients with cancer based on 10 principles for excellence in patient-centered cancer care. The bill of rights is intended "to open a discussion, not be a fait accompli," they emphasize. The document was created with input from peers, cancer patients, caregivers, and survivors. The 10 principles that anchor the bill of rights cover risk reduction, diagnosis, multidisciplinary expertise, treatment, second opinion, coordinated care, communication, supportive and ancillary services, privacy, and follow-up care. Collectively, these principles provide a "starting point for a much larger effort," the editorialists point out. "Closing this gap will require sweeping changes in how our care is reimbursed and in how we manage our time," Jacobson and colleagues write. "It will mean retraining physicians and staff to facilitate true team-based care, and it will demand raised expectations for all cancer clinicians, including how they coordinate care and close communication loops." In an interview, Jacobson acknowledged that the editorial "was meant to be provocative." The authors wanted to send a "clarion call" to cancer care providers telling them that a shift toward "true team-based care" requires more than good intentions, he told Medscape Medical News. "The 10 principles can only be realized by building effective systems of care," Jacobson explained. "We owe it to our patients to have sound, reliable systems of care in place to ensure that high quality and a high-quality patient experience are achieved 100% of the time." The 10 Principles for a Cancer Patient's Bill of Rights A proposed bill of rights for patients with cancer is based on the following goals for patient-centric cancer care excellence: Risk reduction Patients have the right to be educated about opportunities to reduce cancer risk, to be screened to detect cancer early, and to be appropriately counseled on the basis of the findings and the best available evidence. Diagnosis Patients with suspected cancer have the right to timely access to trained subspecialists, to rapid diagnostic testing, and to accurate interpretations of their test results that are shared with all relevant clinicians. Multidisciplinary expertise Patients diagnosed with cancer for which treatment planning may benefit from multidisciplinary discussion have the right to receive such a service. Treatment Patients have the right to balanced information about treatment options that is provided in understandable language and that takes into account their priorities and values. Patients have the right to have all of their questions answered, including candid and ongoing assessment of their prognosis. Patients have the right to receive up-to-date information about, and have access to, relevant clinical trials. Second opinion Patients have the right to seek a second opinion at any time in their cancer course. Coordinated care Patients have the right to have all testing and treatments provided in a timely and coordinated manner. Communication Patients have the right to read communications among the people who provide their care. Supportive and ancillary services Patients have the right to supportive and ancillary services that address cancer-related health problems (emotional sequelae, pain, symptoms, adverse effects) and personal issues (financial management, caregiving support). Patients have the right to counseling and other services to help them transition from active treatment to follow-up care. Privacy Patients have the right to expect that their privacy will be safeguarded by all members of the treatment team and by ancillary staff. Follow-up care Patients have the right to receive a treatment summary at the end of therapy that explicitly describes how their cancer will be observed, what signs or symptoms to look for, and whom to contact as needs arise. A 5-year pilot project launched by the Center for Medicare & Medicaid Innovation shows that it is possible to shift the current paradigm, Jacobson said. The Oncology Care Model incentivizes oncology practices through monthly management payments and potential shared savings. Results demonstrate that this strategy has been effective for reducing hospitalization rates and improving management of end-of-life resources. Other ways to achieve long-lasting results include enhancements to electronic health records. This could go a long way toward improving end-of-life care by initiating difficult conversations with patients with advanced cancer, Jacobson said. Helping practices build more streamlined processes could make it "easy to do the right thing," he added. Conducting regular performance audits of providers that includes feedback could also effect lasting change. Last but not least, said Jacobson, is messaging. He pointed out that messaging is an area in which the American Society of Clinical Oncology (ASCO) could play a major role. "It is really an issue of prioritization," he noted. "Central to our thinking is that patients should understand their rights at the time of cancer diagnosis, that oncologists are in the best position to guide their patients, and that, from an advocacy perspective, ASCO is well positioned to advance a bill of rights," he commented. "We recommend that ASCO bring together a diverse group of stakeholders to create a draft bill of rights. The draft could then be posted for public comment, and based on the feedback, a final bill of rights would be created, published in the Journal of Clinical Oncology, and promoted at the annual meeting and on social media." Kindness in Cancer CareResults from an earlier study, led by a coauthor of the bill of rights, Leonard L. Berry, PhD, MBA, of Texas A&M University, College Station, has demonstrated that in high-pressure settings in which there is too much to do in too little time, compassionate care can reduce the emotional turmoil associated with cancer diagnosis and treatment. It can also potentially improve patient outcomes and prevent physician burnout. There are six different types of kindness embedded in compassionate cancer care, Berry and colleagues say. They include deep listening, empathy, generosity, timely care, gentle honesty, and family caregiver support. The researchers identified these elements following a review of service care at cancer facilities in the United States and Australia. In the process, they interviewed 400 patients, families, oncologists, and cancer care staff. "Kindness can be a life vest in a sea of suffering...especially for seriously ill patients," they write in an article that was published in the Journal of Oncology Practice in 2017. In an interview, Berry said that complex cancer care "has become so difficult that in the hustle and bustle of the day, it is easy to leave kindness at the door. We wanted to present kindness to an oncology audience not as a soft, fluffy construct but defined scientifically, which we did." We wanted to present kindness to an oncology audience not as a soft, fluffy construct but defined scientifically. Berry said he is encouraged by the "many pockets of excellence and innovation in cancer care. But if want to find some of the most humane cancer care in the world, you need look no further than pediatrics," he told Medscape Medical News. Caring for children and families who are frightened has led pediatric oncology doctors and nurses to develop solutions that are "truly clever and kind," Berry noted. At one pediatric cancer center, for example, children were encouraged to put their teddy bear through a mock MRI machine prior to undergoing the procedure themselves. A subsequent study showed that the percentage of children who required an anesthetic prior to an MRI was markedly reduced by what Berry called "this generous act of discretionary effort." In their article, the authors detail the six types of kindness and how they reinforce a compassionate cancer care culture. Deep listening: Taking the time to understand the needs of patients and their families using simple, open-ended questions builds trust and respect, the researchers say. This means asking patients what they understand their prognosis to be and what concerns they have about what lies ahead. At Brigham and Women's Hospital in Boston, Massachusetts, the researchers learned that nurses in the intensive care unit routinely ask patients, "What's the most important thing we can do for you today?" Deep listening helps clinicians avoid "the hidden costs of not listening," the study authors point out. "Deep listening should lead to treatment plans that work better for the values of patient and the family," Berry explained. "You're more likely to get it right if you listen than if you don't." Deep listening is also crucial for patients receiving end-of-life care. For example, through deep listening, a frail, elderly patient with advanced lung cancer was allowed to move from active treatment to therapy that improved his quality of life. The man lived for only 3 months, but in that time he was able to fulfill his lifelong dream of making a family pilgrimage to Mecca. Empathy: Seeing things from the patient's perspective and responding to emotion without judgment is an anticipatory kindness that prevents avoidable suffering, the researchers say. "Any serious illness confers suffering, but a care team can mitigate avoidable suffering by understanding the emotion that diagnosis and treatment evoke, then injecting kindness." For example, at the Peter MacCallum Radiation Center, in Victoria, Australia, anxious children who are anticipating cancer treatment can choose a superhero costume to wear to appointments. Timely care: The heightened emotion that patients experience following a diagnosis of cancer can intensify a sense of urgency for immediate action. Delays in scheduling clinic appointments and treatment dates or in getting test results can fuel uncertainty and feelings of powerlessness. For parents of children with cancer, getting a treatment plan and developing a routine "greatly reduces stress and anxiety," the researchers say. "The sooner we can give them the information they need, the more they can calm down," commented one oncologist who was interviewed. The review also showed that some cancer centers are investing in ways to improve the timeliness of care, despite the added upfront costs. Some examples include the following:
Increasing the opportunities for patients to receive information through an e-learning website and appropriate cancer services via telemedicine and videoconference visits could also improve the timeliness of effective care, the researchers note. Gentle honesty: In an interview, one oncologist remarked that patients and their doctors are overly optimistic "far too often." Patients and doctors need a healthy dose of reality to make good decisions, he explained. When cancer is advanced and remission unlikely, clinicians should convey the truth directly using well-chosen words that guide patients towards "intrinsic hope," the study authors write. Intrinsic hope "can emerge unexpectedly after longed-for outcomes fail to materialize and focused hope fades," notes a BMJ blog on the topic, which also notes that "as opposed to outer-directed focused hope, intrinsic hope centers on subjective, personal issues." As an example, the study authors suggest that, with pain well-managed, patients can choose to spend a good day as they want — surrounded by loving family, with a small grandchild or a pet on their lap. Support for Family Caregivers: Studies show that family caregivers need support to provide care for a loved one and to stay healthy themselves. "If a family is well prepared to take care of a cancer patients during treatment, this helps them cope too," said Berry. At the Johns Hopkins Kimmel Cancer Center in Washington, DC, a free, annual 3-day offsite weekend retreat for women with metastatic breast cancer and their partners provides information and resources and an opportunity to meet other patients. Individual kindness can be equally powerful, according to Rana L. A. Awdish, a study coauthor and medical director of the pulmonary hypertension program at the Henry Ford Hospital in Detroit. She recalls how in 2008 she experienced a ruptured occult adenoma of the liver that resulted in multiorgan failure and the loss of her pregnancy. While in intensive care at Henry Ford, her husband slept at her bedside. "Radiation technicians would gently cover him in a leaded apron when they shot my x-ray, rather than disrupt his sleep," Awdish recalls. "That silent awareness of his needs was so simple and yet meant everything to us. It meant his suffering was seen." However, while enduring five major surgeries and multiple hospitalizations, Awdish also experienced care that seemed casual and indifferent. At times, there was a complete lack of empathy, she wrote in a 2017 article in the New England Journal of Medicine.Subsequently, she championed communications training that would allow health professionals at Henry Ford Hospital to speak to their patients more effectively and empathetically. Kindness to Self to Prevent BurnoutIn spite of clinicians' good intentions, the demands of complex clinical care along with financial and institutional stressors can act as barriers to compassionate care, Berry commented in the interview. Paradoxically, physicians who get past the barriers often succumb to "compassion fatigue." So what can oncologists do to prevent burnout? "Oncology is an especially challenging profession," said Berry. "There is no magic bullet." The best things that clinicians can do "all fall under the heading 'take care of yourself,' " he said, adding, "If you don't, you will slowly lose your ability to care for others." Kindness is not only an important antidote to the stress that patients and families feel, but it also serves as a buffer between clinicians and the stressors they experience, he pointed out. Kindness can help preserve resilience when clinicians are feeling exhausted or a patient suffers a setback. "Compassionate physicians do suffer," said Berry. "It's not easy to go into an exam room with lab results that indicate the chemo is not working and you have to tell the patient who has been waiting for 4 days that their tumor is growing." Clinicians should work as a team to discuss difficult cases and share support offline, he suggested. Periodic peer group sessions with other clinicians — perhaps led by another oncologist, a chaplain, or a psychiatrist — will reinforce the fact that clinicians are not alone in the work they do. Carve out time for rewarding nonclinical activities, such as teaching or tackling a research project. Working with other people, learning from them, getting a paper published, and giving a talk at a symposium are all ways to maintain equilibrium, said Berry. Anyone with a stressful life needs to make time for regular physical exercise, and doctors are no exception. However, most doctors do not make time to exercise, Berry commented. He recommends getting outside ― even a 10-minute walk can help reset one's perspective ― as well as reflecting on the importance of one's work, or an upcoming vacation with one's family, or time spent with a grandchild. "It is such a powerful antidote to all things stressful in life," he pointed out. The bill of rights research was funded by AstraZeneca. Jacobson has disclosed no relevant financial relationships. Other authors' relevant financial relationships are listed on the journals' website. J Oncol Prac. Published online on February 10, 2020. Full text For more from Medscape Oncology, follow us on Twitter: @MedscapeOnc. |
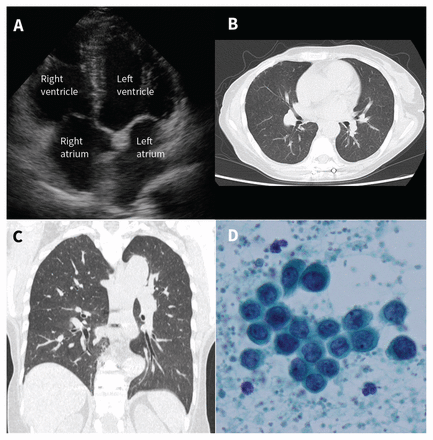
| Pulmonary tumour thrombotic microangiopathy - CMAJ Posted: 08 Mar 2020 09:34 PM PDT A 69-year-old woman with metastatic breast cancer presented to the hospital with a 5-day history of progressive dyspnea. On examination, the patient had tachycardia and an oxygen saturation level of 91% while breathing ambient air. An electrocardiogram showed an S1Q3T3 pattern. Transthoracic echocardiography found a dilated right atrium and ventricle (Figure 1A). Computed tomography of her chest showed diffuse centrilobular granular shadows without pulmonary thromboembolism (Figures 1B and 1C). Figure 1: (A) Transthoracic echocardiography in a 69-year-old woman with metastatic breat cancer, showing a dilated right atrium and right ventricle. Computed tomography (CT) scans of the chest showing (B) axial and (C) coronal views of diffuse centrilobular granular shadows in both lungs. (D) Blood sample obtained from the pulmonary artery showing adenocarcinoma (Papanicolaou stain, original magnification × 40). Considering the patient's medical history, we suspected pulmonary tumour thrombotic microangiopathy because of the signs of right heart failure on electrocardiography and echocardiography, without any signs of pulmonary thromboembolism on computed tomography. Right-heart catheterization showed pulmonary hypertension with a mean pulmonary artery pressure of 34 (normal mean value 14 [SD 3]) mm Hg. We obtained cytology by performing pulmonary wedge aspiration, in which a Swan–Ganz catheter was placed in the pulmonary artery wedged position and blood was gently withdrawn from the catheter. Cytology indicated adenocarcinoma (Figure 1D). We diagnosed pulmonary tumour thrombotic microangiopathy due to metastatic breast cancer. The patient underwent chemotherapy but died 2 weeks after admission to hospital. Pulmonary tumour thrombotic microangiopathy is defined as a nonoccluding pulmonary tumour embolism accompanied by fibrocellular intimal proliferation of small pulmonary arteries, eventually leading to stenosis and occlusion of the pulmonary arteries.1 This condition is an underrecognized cancer-related complication, causing fulminant pulmonary hypertension and mimicking pulmonary thromboembolism. The incidence rate is 0.9%–3.3% in patients with carcinoma at autopsy,1,2 and the most common site for the primary tumour is the stomach, followed by lung and breast.1 Chemotherapy may alter the prognosis.2 Although pulmonary tumour thrombotic microangiopathy is seldom diagnosed before death, pulmonary wedge aspiration cytology is a simple and useful method for diagnosis at an early stage.3 Footnotes
|
| You are subscribed to email updates from "pulmonary hypertension prognosis" - Google News. To stop receiving these emails, you may unsubscribe now. | Email delivery powered by Google |
| Google, 1600 Amphitheatre Parkway, Mountain View, CA 94043, United States | |
Comments
Post a Comment