Overactive enzyme causes hereditary hypertension - Medical Xpress
Overactive enzyme causes hereditary hypertension - Medical Xpress |
- Overactive enzyme causes hereditary hypertension - Medical Xpress
- Cholesterol and COVID-19; Novel HFpEF Drug; Full Speed on CV Surgery? - MedPage Today
- Information About COVID-19 for Pulmonary Hypertension Patients - Pulmonary Hypertension News
| Overactive enzyme causes hereditary hypertension - Medical Xpress Posted: 11 Jun 2020 06:00 AM PDT 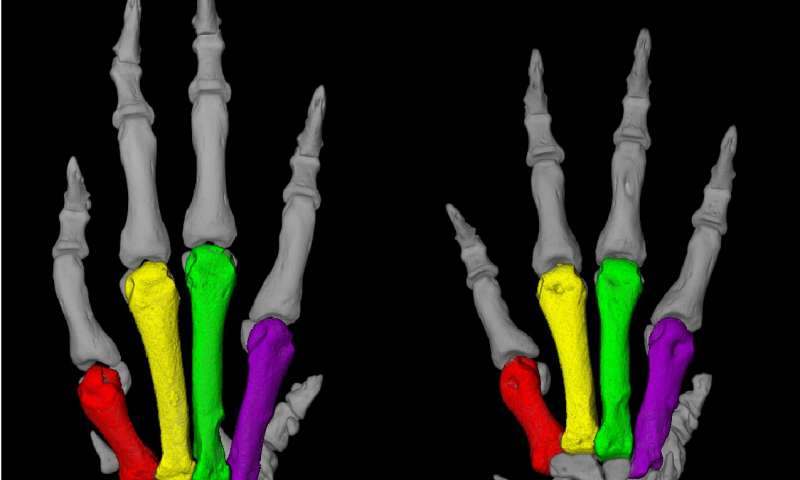 A Turkish family from a village near the Black Sea caught the attention of medical researchers in the early 1970s, when a physician discovered that many members of this large family had both unusually short fingers and astronomically high blood pressure, sometimes twice as high as that of healthy people. Those affected die around the age of 50, usually due to a stroke. Some 20 years later, a group of researchers at the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), led by Professor Friedrich Luft and Dr. Sylvia Bähring, began to study this mysterious phenomenon. It proved to be no easy task. Not until May 2015 were the researchers able to report in the journal Nature Genetics that they had found an altered gene in all patients who were affected by the hypertension and brachydactyly (HTNB) syndrome—i.e., high blood pressure and abnormally short digits. The genetic disorder is also known as Bilginturan syndrome, after its Turkish discoverer. The genetic makeup encodes an enzyme called phosphodiesterase 3A, or PDE3A for short, that regulates both blood pressure and bone growth. The gene mutation that Luft and his team had discovered causes the enzyme to be more active than usual. Researchers provide the missing evidence There was no evidence that definitely shows that the mutated PDE3A causes Bilginturan syndrome, which has since been discovered in other families around the world. An international group of 40 researchers from Berlin, Bochum, Limburg, Toronto (Canada) and Auckland (New Zealand) has now supplied this evidence in the journal Circulation. Participating in the study were research groups from the MDC and Charité - Universitätsmedizin Berlin, including teams led by Professors Luft, Michael Bader, Maik Gollasch and Dominik N. Müller as well as Dr. Arndt Heuser and Dr. Sofia Forslund. The last author of the paper is Dr. Enno Klußmann, head of the MDC's Anchored Signaling Lab. "We mainly worked with two animal models," reports Dr. Lajos Markó, the paper's co-lead author along with Maria Ercu. One of the models consisted of genetically modified mice in which the human enzyme PDE3A in the smooth muscle cells of the vessel walls was overactive due to the gene alteration. "These animals exhibited extremely high blood pressure as compared to the control animals," Markó says. 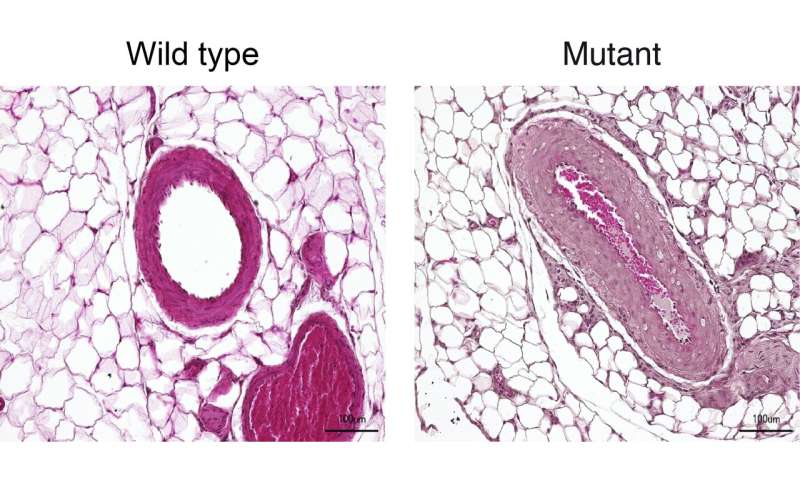 Genetically modified rats recapitulate the genetic disorder But what proved more interesting to the scientists was a rat model created by the Bader Lab using CRISPR-Cas9 technology. With the help of the gene-editing tool, the team had altered nine base pairs in a region of the PDE3A gene that is mutated in the syndrome, a so-called mutation hot spot. The resulting enzyme differed from the normal variants with respect to three amino acids. "And just as in the patients, this tiny change increased the activity of the enzyme," Ercu says. "The rats resembled human patients to a truly extraordinary degree," Ercu adds. "They not only suffered from high blood pressure, but the toes on their forefeet were significantly shortened—similar to the fingers of people with the syndrome." And using micro-computed tomography, the researchers discovered a prominent loop in the brain vessels of the rats that is also found in people with the syndrome. "Our rat model provides, in my view, definitive proof that the syndrome is caused by a mutation in the PDE3A gene," Klußmann says. The researchers have even developed an approach for treating this inherited form of high blood pressure. "There is a drug called riociguat that is already approved as a therapeutic for pulmonary hypertension," Klußmann says. It activates an enzyme that produces a signaling molecule, which in turn dampens down an overactive PDE3A. "The blood pressure of rats to which we administered a derivative of riociguat dropped to a normal level," Klußmann reports. There are already other PDE3A inhibitors on the market, but they are not suitable for long-term therapy due to their side effects. Klußmann now wants to take a closer look at how the mutated PDE3A interacts with other protein molecules. Stronger interaction with certain adaptor proteins, he says, could cause cells of the vessel walls to replicate at an increased rate. In fact, Klußmann has a big goal in his sights: "By learning more about the effects of the PDE3A's interactions with other proteins and understanding how they are involved in the regulation of blood pressure, we will hopefully find new and more effective therapeutic approaches for one of the most widespread diseases of all, hypertension." Provided by Max Delbrück Center for Molecular Medicine Citation: Overactive enzyme causes hereditary hypertension (2020, June 11) retrieved 11 June 2020 from https://medicalxpress.com/news/2020-06-overactive-enzyme-hereditary-hypertension.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only. |
| Cholesterol and COVID-19; Novel HFpEF Drug; Full Speed on CV Surgery? - MedPage Today Posted: 09 Jun 2020 11:31 AM PDT  High cholesterol in tissue may increase entry points into cells for SARS CoV-2, the virus that causes COVID-19, such that rapidly dropping cholesterol in the blood could increase risk, researchers found in preclinical experiments reported on the preprint server bioRxiv, which is not peer reviewed. The Pittsburgh Post-Gazette has an explainer. ST-segment-elevation MI admissions have dropped by an average 50%, with about half presenting later than usual, according to an international survey by the European Society of Cardiology. The first molecular profile comparing blood samples of people with and without COVID-19 showed that differences fell into two groups -- those related to the immune system and those related to platelet function. (Cell, NIH Director's Blog) Antihypertensives, anticoagulants, antiplatelets, statins ... there's a long list of heart drugs being studied for COVID-19, as the Wall Street Journal notes. (subscription required) No drop in ACE inhibitor or angiotensin receptor blocker prescriptions was seen during the early part of the pandemic in the U.S., according to a study of pharmacy data in JAMA. (New York Times) Novel calcium sensitizer levosimendan missed the primary endpoint in its phase II trial for treating pulmonary hypertension and heart failure with preserved ejection fraction (HFpEF), although there were some significant benefits when results were parsed a different way and for 6-minute walk distance, Tenax Therapeutics announced. Interpretation of trials may take a pre- and post-COVID-19 analysis, along with other accommodations, according to a consensus document on heart failure trials from the European Society of Cardiology's Heart Failure Association. (European Heart Journal) The Montreal Heart Institute kept right on doing cardiac surgeries through the COVID-19 pandemic with a 2% or less rate of patients contracting the virus, using strategies it says others can replicate. (CBC) A hospital in India allegedly tried to pass off a COVID-19 death as a heart attack. (Cardiovascular Business) Centers for Medicare & Medicaid Services issued new billing codes for intravascular lithotripsy in peripheral arteries done in hospital outpatient and inpatient settings. (Shockwave Medical) European approval of a generic of apixaban (Eliquis) was recommended by an advisory committee. The shortest dual antiplatelet regimen yet -- 1 month -- was approved by European regulators for the Resolute Onyx drug-eluting stent, Medtronic announced. A packed emergency department is more likely to lead to cardiac arrest death, researchers reported in Critical Care. An air filter for the home likely does improve blood pressure, a meta-analysis concluded in Hypertension. |
| Information About COVID-19 for Pulmonary Hypertension Patients - Pulmonary Hypertension News Posted: 19 Mar 2020 01:12 AM PDT UpdatesEditor's Note: This page is updated weekly with new information related to COVID-19 testing and potential treatments in development. Click the arrow next to the date to expand the text. ► June 10, 2020 Gilead Sciences has filed for approval in the European Union for remdesivir to treat COVID-19, according to the European Medicines Agency, which received a conditional marketing authorization application for the treatment. Because some of the data were already submitted under a rolling review, the agency expects to complete its review of the data under a shortened timeline. If the information submitted is deemed sufficient, the agency will work with the European Commission to potentially fast-track approval of the therapy. Hydroxychloroquine provides no benefit to patients with COVID-19, according to preliminary results of the Phase 2/3 RECOVERY clinical trial (NCT04381936). The randomized study, which enrolled more than 11,000 patients across the U.K. and is testing a variety of therapies to treat the virus, found that the antimalarial showed no beneficial effect on the length of hospital stays nor on other disease outcomes. Based on these results, the investigators made the decision to stop enrolling patients in the hydroxychloroquine arm of the trial. As the controversy over hydroxychloroquine continues, The Lancet journal recently retracted the article, "Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis." The study's results showed there was no benefit to hydroxychloroquine or chloroquine treatment on hospital outcomes for COVID-19, but three of the paper's authors made the decision to retract based on the fact that they had failed to complete an independent audit of the data underpinning their analysis. Because of this, they wrote in a statement for The Lancet, they "can no longer vouch for the veracity of the primary data sources." Brazil's Ministry of Health approved clinical trials of AstraZeneca and Oxford University's COVID-19 vaccine candidate, making it the first country outside the U.K. to do so. The vaccine candidate, known as both ChAdOx1 nCoV-19 and AZD1222, is one of the first to move into Phase 2 testing. Reuters has reported that this vaccine will simultaneously enter both Phase 2 and Phase 3 trials to speed its development. The first round of trials, conducted by Federal University of São Paulo and funded by Brazil's Lemann Foundation, will test the vaccine in 2,000 volunteers who have not previously contracted COVID-19. Inovio will begin testing its COVID-19 vaccine candidate, INO-4800, in a Phase 1/2 trial in South Korea later this month in collaboration with the International Vaccine Institute and Seoul National University Hospital. The trial will assess the vaccine's safety, tolerability, and ability to produce an immune response in 40 adults, ages 19 to 50 years. Inovio plans to expand this trial to include an additional 120 people ages 19–64. Eli Lilly has partnered with Shanghai-based biotech Junshi Biosciences to begin testing a second potential COVID-19 vaccine. Similar to Eli Lilly's first candidate, known as LY-CoV555, JS016 is also an engineered antibody that showed promise in preclinical studies in neutralizing the SARS-CoV-2 virus, which causes COVID-19. The first healthy volunteer in a randomized Phase 1 trial was recently dosed at Huashan Hospital affiliated with Fudan University in China. AbbVie has partnered with Harbour BioMed, Utrecht University, and Erasmus Medical Center to develop 47D11, a monoclonal antibody therapy for COVID-19. AbbVie will support its partners through preclinical activities while preparing for later-stage preclinical and clinical development. Under the terms of their agreement, AbbVie will also have the option to exclusively license the antibody for worldwide therapeutic development and commercialization. Australian biotech CSL, along with the University of Queensland (UQ) and the Coalition for Epidemic Preparedness Innovations (CEPI), have joined forces to accelerate the development of a COVID-19 vaccine candidate first developed by UQ. CSL and CEPI will fund industrial-scale manufacturing of potentially 100 million vaccine doses by the end of 2021, should the product win approval. Preliminary research shows that AstraZeneca's blood cancer treatment Calquence (acalabrutinib) provides a benefit to hospitalized COVID-19 patients. Of 19 severe COVID-19 patients treated with Calquence, 11 who had needed supplemental oxygen no longer needed it, and four of eight patients on mechanical ventilation were successfully taken off. The company said the positive results support the launch of a global Phase 2 trial, which was announced in April. FibroGen has enrolled the first patient in a Phase 2/3 trial in Italy to evaluate pulmonary fibrosis treatment pamrevlumab for severe COVID-19. The trial will assess the effect of pamrevlumab on blood oxygenation in 68 hospitalized patients who will receive either pamrevlumab or standard care. The company also is planning two Phase 2 trials in the U.S. to assess pamrevlumab versus standard care in patients with severe COVID-19. ► June 3, 2020 Gilead announced top-line results from its Phase 3 SIMPLE clinical trial (NCT04292899) testing the safety and antiviral activity of remdesivir. According to the results, patients who received remdesivir treatment for five days were 65% more likely to improve clinically at day 11 than those who received standard care. A preliminary report from 1,059 patients participating in another Phase 3 trial called ACTT (NCT04280705), assessing the effects of remdesivir for the treatment of COVID-19, was published in The New England Journal of Medicine. Early results show that remdesivir was superior to a placebo in shortening the time to recovery in adults hospitalized with COVID-19 and lower respiratory tract infection. With early data showing that redemsivir may speed up recovery time, certain U.K. patients hospitalized with COVID-19 who meet specific criteria will soon be able to access the investigational therapy. Patients will be selected based on expert clinical advice for greatest potential benefit. The plan is supported by the U.K. government, Gilead, the National Health Service, and the Medicines and Healthcare Products Regulatory Agency. Remdesivir has not yet been approved for any indication. BerGenBio has dosed the first patient in its Phase 2 trial testing oral kinase inhibitor bemcentinib, along with standard care, in hospitalized COVID-19 patients. The treatment candidate is the first to be selected as part of the U.K.'s Accelerating COVID-19 Research & Development (ACCORD) platform, an initiative designed to rapidly test potential COVID-19 therapies in early trials before sending them into large-scale studies. The trial is recruiting an estimated 120 participants at eight sites across the U.K. The first patients have been dosed in a Phase 1 trial (NCT04411628) led by Lilly and AbCellera to test an antibody treatment against COVID-19 called LY-CoV55. This marks the world's first in-human study evaluating an antibody for treating the virus. The randomized, single ascending dose trial is evaluating the safety, tolerability, pharmacokinetics (movement in the body), and pharmacodynamics (effect on the body) of LY-CoV555 administered intravenously in patients hospitalized with COVID-19. The trial, which will enroll an estimated 40 participants, is currently open for recruitment at multiple sites in the U.S. It is expected to be completed on Aug. 23. An investigational antiviral antibody being developed by Celltrion for COVID-19 has shown positive results in a preclinical study, the company announced. The study tested the efficacy of a high and low dose of the treatment candidate in an animal model. Results showed that the therapy led to a hundredfold reduction in viral load and reduced lung lesions to a normal level. Advent has manufactured 13,000 doses of AZD1222, AstraZeneca's experimental COVID-19 vaccine, for use in clinical testing. According to the Italy-based manufacturing company, it delivered 4,000 doses to the University of Oxford for its Phase 2/3 COV002 trial (NCT04400838), which is aimed at testing the potential vaccine in more than 10,000 healthy volunteers. It is not yet open for recruitment. Massachusetts Eye and Ear and Massachusetts General Hospital have partnered with AveXis to develop a new potential genetic vaccine, called AAVCOVID, against COVID-19. The hospital will conduct safety and efficacy testing in preclinical studies, while AveXis will start manufacturing the vaccine. It will use a gene therapy approach, where a harmless adeno-associated virus will deliver a genetic code to human cells to make portions of SARS-CoV-2, the virus that causes COVID-19, to elicit an immune response. PureTech will begin human clinical trials to test its experimental treatment LYT-100 (deupirfenidone) in people with respiratory complications following recovery from COVID-19 infections. LYT-100, an analog of the pulmonary fibrosis treatment Esbriet (pirfenidone) is currently being tested in a Phase 1 trial (NCT04243837) in healthy volunteers and patients with breast cancer-related lymphedema for safety, tolerability, and pharmacokinetics. ► May 27, 2020 Results from the first potential COVID-19 vaccine to be tested in a Phase 1 clinical trial (NCT04313127) were recently published in the journal The Lancet. They showed that the adenovirus type 5 vectored COVID-19 (Ad5-nCoV) vaccine was tolerable and able to trigger an immune response as early as 14 days after vaccination. The researchers concluded that the potential vaccine is worth further investigation but cautioned that they are still a long way off from having a COVID-19 vaccine available to all. Novavax is enrolling the first participants in a Phase 1/2 trial testing its vaccine candidate, NVX‑CoV2373. The trial is being conducted in two parts, the first of which is taking place in Australia and is testing the safety and immune response in healthy volunteers, ages 18 to 59. The Phase 2 portion will be conducted in multiple countries and will include a broader age range to test the vaccine's safety, immunity, and ability to reduce COVID-19 disease. Glenmark Pharmaceuticals is launching a Phase 3 trial in India to test if early treatment with favipiravir and umifenovir improves the efficacy of the antiviral combination in hospitalized COVID-19 patients. The trial is expected to enroll approximately 158 participants with moderate COVID-19, who will be randomized to receive favipiravir either with or without umifenovir, plus standard care. Covis Pharma has announced the start of a Phase 3 trial (NCT04377711) to assess the safety and efficacy of the asthma medication Alvesco (ciclesonide) in people with symptoms of COVID-19, ages 12 and older who are not hospitalized. Researchers think the treatment may suppress viral replication and alleviate symptoms of the disease. The multicenter, randomized trial is expected to recruit 400 participants across the U.S. and is estimated to be completed by the end of the year. A new trial (NCT04303507) led by the University of Oxford and the Mahidol Oxford Tropical Medicine Research Unit in Thailand is recruiting participants to test the effect of chloroquine and hydroxychloroquine on COVID-19. The randomized trial will enroll an estimated 40,000 healthcare workers across Europe, Africa, Asia, and South America who have had close contact with patients with either proven or suspected COVID-19. There is currently no conclusive proof that either chloroquine or hydroxychloroquine can prevent or treat COVID-19. Octapharma is launching a Phase 3 trial to test whether Octagam 10%, its intravenous immune globulin therapy, can slow or prevent respiratory deterioration in COVID-19 patients.The multicenter, randomized trial will enroll 54 patients with severe COVID-19 at approximately 10 sites in the U.S. Octagam 10% is an approved treatment for chronic immune thrombocytopenic purpura in adults. IMV has selected a vaccine candidate against COVID-19 that it plans to advance into in-human studies. The vaccine, called DPX-COVID-19, is based on peptide epitopes that showed robust immune and antibody responses in preclinical studies. The company anticipates launching a Phase 1 trial to test the potential vaccine this summer. AstraZeneca has finalized agreements for the first 400 million doses of its vaccine candidate being developed by the University of Oxford. It has already secured manufacturing capacity for 1 billion doses and plans to begin the first deliveries in September. The vaccine, called AZD1222, (previously ChAdOx1 nCoV-19) is being tested in a Phase 1/2 trial (NCT04324606) to determine the safety, efficacy, and immune response in more than 1,000 healthy volunteers. ► May 20, 2020 Moderna announced positive interim results from a Phase 1 clinical trial (NCT04283461) testing its potential COVID-19 vaccine mRNA-1273. The trial, led by the National Institutes of Health, is testing three different doses of mRNA-1273 in healthy volunteers ages 18–55 for safety, immune response, and adverse effects. The results so far show that the vaccine is generally safe and well tolerated. Moderna is planning a Phase 2 trial to further test the potential vaccine and determine the right dose to be used in pivotal studies expected to start in July. The Phase 1 trial is still recruiting healthy volunteers in Georgia, Maryland, and Washington. NeuroRx is sponsoring a Phase 2b/3 trial (NCT04360096) that will test aviptadil in critically ill COVID-19 patients. The goal of the trial is to identify COVID-19 patients with non-acute lung injury and treat them with aviptadil in hopes of preventing progression from non-acute to acute lung injury and acute respiratory distress syndrome, or ARDS, both complications associated with COVID-19. The multicenter, randomized, placebo-controlled trial will recruit approximately 144 participants with COVID-19 and evidence of lung injury. Enrollment is not yet open. Stanford Medicine is currently enrolling participants in a Phase 2 trial (NCT04331899) testing whether peginterferon lambda-1a can help patients to recover faster and limit viral shedding, which lowers the risk of transmission to others. The open-label, randomized trial is recruiting an estimated 120 participants in California, ages 18 to 64, with an initial diagnosis of COVID-19 and symptoms of respiratory infection without respiratory distress. Peginterferon lambda-1a is an antiviral that has already been tested against viruses that cause hepatitis. The University of Oxford has launched a trial in the U.K. that will test the ability of existing treatments such as hydroxychloroquine and azithromycin to prevent, slow, or even halt the progression of COVID-19 and reduce the severity of its symptoms, avoiding hospital admissions in people older than 50. The FDA completed its review of ViralClear's investigational new drug application for merimepodib to treat adults with advanced COVID-19. This means the company can now start its proposed Phase 2 trial testing the potential treatment. The trial will be randomized, double blind, and placebo-controlled and is expected to be conducted at multiple sites across the U.S. It will test the safety and efficacy of merimepodib in adults infected with SARS-CoV-2 and admitted to the hospital requiring supplemental oxygen. Interim results from a multicenter, randomized trial in Russia testing Avigan (favipiravir) showed that 60% of the 40 patients with COVID-19 who received the treatment tested negative for the virus after five days, according to the Russian Direct Investment Fund, which is financing the study. The trial is expected to include 330 participants with confirmed COVID-19 at sites across Russia. Avigan is an antiviral first developed in Japan for the treatment of influenza. Results from an observational study in France show that hydroxychloroquine does not significantly lower admissions to intensive care or death in patients hospitalized with pneumonia due to COVID-19. In addition, a randomized trial in China found that patients with mild to moderate COVID-19 treated with the anti-malarial drug did not clear the virus quicker than those receiving standard care. More adverse events were also recorded in patients treated with hydroxychloroquine. Findings from both studies, published in The BMJ journal, add to growing evidence that hydroxychloroquine may not be effective against COVID-19. ► May 13, 2020 Moderna's investigational mRNA vaccine for COVID-19, called mRNA-1273, was awarded the FDA's fast-track designation, the first to receive this status, which is intended to speed up the review time for a potential medication. The company anticipates launching a Phase 3 trial testing the vaccine in early summer. An observational study conducted by the University of Albany and the New York State Department of Health found no benefit to treating COVID-19 patients with hydroxychloroquine. This is at least the third such study to reach this conclusion, according to the FDA. After reviewing the medical records of 1,438 patients from 25 hospitals in the greater New York area, the researchers found that treatment with hydroxychloroquine, azithromycin, or both did not significantly lower the in-hospital death rate. The study was published in the Journal of the American Medical Association. Novartis and Incyte are rolling out a second Phase 3 trial to test the safety and efficacy of Jakafi (ruxolitinib) compared with standard care in patients with COVID-19 who develop acute respiratory distress syndrome. Jakafi is a prescription medication for polycythemia vera, myelofibrosis, and acute graft-versus-host disease. The trial will be conducted in the U.S. The companies had previously announced a Phase 3 trial called RUXCOVID, which is examining Jakafi's effectiveness in treating COVID-19-associated cytokine storm. That trial is currently underway. A new Phase 2 trial is being planned to investigate the effectiveness of remdesivir with Olumiant (baricitinib) in treating COVID-19. It is expected to enroll up to 1,000 participants at 100 sites worldwide. Enrollment has already begun in the U.S. Remdesivir is an investigational broad-spectrum antiviral being developed by Gilead. It is not yet approved anywhere in the world for any indication. Olumiant is an anti-inflammatory by Lilly used to treat some patients with rheumatoid arthritis. The trial, being sponsored by the National Institute of Allergy and Infectious Diseases, follows on the heels of another study that found that remdesivir on its own shortened patients' recovery time over a placebo. A Phase 2 trial (NCT04276688) conducted in China tested a combination of three antivirals, Kaletra (lopinavir-ritonavir), an HIV antiviral, ribavirin, a hepatitis C treatment, and interferon beta-1, a treatment used in multiple sclerosis. The trial, completed on March 31, showed that the combo therapy suppressed the virus at day seven. This was five days earlier than in patients who were treated with Kaletra only. The combo was also found to be safe, leading to minor gastrointestinal side effects such as diarrhea and vomiting. The FDA granted emergency use authorization for a new laboratory test kit to detect SARS-CoV-2. The kit, called OPTI SARS-CoV-2 RT-PCR, can detect the genetic material of the virus in oral and nasal swabs as well as sputum. ► May 6, 2020 The U.S. Food and Drug Administration has granted Gilead Sciences' remdesivir emergency use authorization for the treatment of COVID-19, the company recently announced. The agency's decision was based on available data from two global Phase 3 clinical trials. Top-line results from one of these Phase 3 trials, known as SIMPLE (NCT04292899), showed that a shorter, five-day course of treatment with remdesivir leads to similar improvements in clinical status as a 10-day course of treatment in patients hospitalized with severe COVID-19. The open-label trial is testing the effects of two dosing durations (five days and 10 days) of the experimental antiviral. The European Medicines Agency also began a rolling review of remdesivir for COVID-19, with the goal of shortening the review time of the treatment from months to weeks. The FDA granted emergency use authorization to a new antibody test for COVID-19. The test, developed by Roche, is called Elecsys. It is able to determine with 100% sensitivity and 99.8% specificity whether a person has been exposed to SARS-CoV-2 in the past and developed antibodies against the virus. The first participants have been dosed in a Phase 1/2 trial in the U.S. that is testing four mRNA vaccine candidates being jointly developed by BioNTech and Pfizer against COVID-19. The first part of the trial is a dose escalation stage that is enrolling up to 360 healthy individuals. The BNT162 vaccine program also includes a similar trial in Germany, which completed dosing of its first group last week. Massachusetts General Hospital is conducting a clinical study to assess the potential efficacy of inhaled nitric oxide to reverse hypoxemia (abnormally low oxygen levels in the blood) in patients with severe COVID-19. Mallinckrodt Pharmaceuticals is providing both funding and INOmax — nitric oxide gas for inhalation — for the study. Patients are being enrolled in a Phase 2 trial at Beaumont Hospital in Royal Oak, Michigan, to test a combination of naltrexone and ketamine for treating COVID-19. Researchers will assess whether reducing inflammation with the combo therapy may lessen the severity of COVID-19 symptoms. AstraZeneca and the University of Oxford are collaborating on developing a potential vaccine against COVID-19 to be developed and distributed globally. The potential vaccine, called ChAdOx1 nCoV-19, is being developed by the University of Oxford and will be manufactured and distributed by AstraZeneca. ► April 29, 2020 German regulatory authority Paul-Ehrlich-Institut approved a Phase 1/2 clinical trial for BioNTech and Pfizer's BNT162 vaccine program, which is testing four mRNA vaccine candidates to prevent COVID-19. In the first phase of the trial, researchers will test the vaccines in 200 healthy volunteers ages 18 to 55 to determine the optimal dose. The second part will include participants at a higher risk of severe COVID-19. Asthma medication MN-166 (ibudilast) will be tested in a clinical trial to assess its potential in treating acute respiratory distress syndrome caused by COVID-19. The trial is a collaboration between Yale University and pharmaceutical company MedicNova. A trial called STOP-COVID19 is expected to start recruiting up to 300 hospitalized patients with COVID-19 in the U.K. in May to test the experimental treatment brensocatib. Insmed, which is developing the treatment for bronchiectasis and other inflammatory diseases, will provide funding and the medication for the trial, which is sponsored by the University of Dundee. A new trial funded by Amazon and run by Columbia University will assess whether plasma (the liquid portion of the blood devoid of cells) obtained from COVID-19 survivors can be used to prevent SARS-CoV-2 infections or treat COVID-19. The trial aims to recruit 450 people who have been in close contact with COVID-19 patients such as healthcare workers as well as intensive care unit patients. Astra Zeneca will test the diabetes treatment Farxiga (dapagliflozin) in a trial to assess its potential to decrease the risk of death from serious complications and organ failure in COVID-19 patients. The trial, which is a collaboration with Saint Luke's Mid America Heart Institute, will enroll 900 participants in the U.S. and European countries that are experiencing high rates of COVID-19 cases. An experimental treatment by Vivacelle Bio will be made available for compassionate use in COVID-19 patients. The experimental treatment, called VBI-S, is currently being tested in a Phase 2 trial (NCT04257136) people with reduced blood circulation due to sepsis. Genalyte launched a COVID-19 test to detect antibodies produced by the body against SARS-CoV-2. Cleared by the FDA, the test runs on Genalyte's Maverick platform. Quest Diagnostics started offering an antibody testing service for COVID-19 on blood samples. The antibody test, intended for healthcare providers, can determine whether a person has antibodies in their blood against SARS-CoV-2, meaning they have been exposed to the virus and may have some level of immunity against it. ► April 22, 2020 Biogen, Broad Institute, and Partners HealthCare are launching a consortium to build and share a COVID-19 biobank. The biobank will contain blood samples of Biogen employees willing to participate in the project who contracted and have recovered from COVID-19. People identified as close contacts of those individuals are also eligible to participate regardless of whether they were confirmed to have COVID-19. The biobank will help scientists understand why some people infected with SARS-CoV-2 become seriously ill while others do not even show any symptoms. A better understanding of the biology of the virus and the response the human body has to it will help speed the quest for potential vaccines and treatments. Alexion is planning a Phase 3 trial testing Ultomiris (ravulizumab-cwvz) for treating severe COVID-19. The global trial will include approximately 270 hospitalized patients who have severe pneumonia or acute respiratory distress syndrome. Ultomiris is FDA-approved for paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome (aHUS). In the coming weeks, Novartis is planning to start enrollment for its randomized Phase 3 trial testing hydroxychloroquine in hospitalized COVID-19 patients. An estimated 440 patients will be recruited across more than a dozen U.S. sites. Sinovac has launched a randomized Phase 1 trial in China to test its vaccine candidate against COVID-19. The first group of an estimated total of 144 healthy adults have already been given the first dose of two doses of the experimental vaccine. The FDA authorized a saliva test for emergency use to diagnose COVID-19. The test was developed by Rutgers University in collaboration with other groups. The test can only be used in a healthcare setting and is not available to purchase for home testing. LabCorp has received emergency use authorization from the FDA for the first diagnostic test using samples collected from people at home. The at-home kit includes a special cotton swab to collect a sample from the individual's nose. The sample is then mailed in an insulated package to a LabCorp lab. The products contained in the LabCorp collection kit are the only ones authorized for at-home use at this time. ► April 15, 2020 Biohaven Pharmaceuticals will soon start a Phase 2 clinical trial to test intranasal vazegepant in treating lung complications caused by COVID-19. The trial will run in collaboration with Thomas Jefferson University and other institutions. The FDA has cleared a Phase 2 trial that will test the safety and efficacy of Hope Biosciences' adipose-derived mesenchymal stem cells to provide immune support against COVID-19. The trial will enroll an estimated 100 front-line healthcare workers and first responders and will test three doses of cells over 14 weeks. Vaxil has completed the first preclinical experiment testing its COVID-19 vaccine candidate. According to the results, the vaccine candidate showed a favorable immune response in healthy donor blood cells. The company has now begun the next set of experiments to better understand the immune responses and determine dosing, with additional experiments planned. Sanofi and GlaxoSmithKline are partnering on developing an adjuvanted COVID-19 vaccine. Sanofi is providing its S-protein COVID-19 antigen, based on a recombinant RNA technology that created an exact match to proteins found on the surface of the virus. GSK will contribute its pandemic adjuvant technology, which is intended to boost the immune response and may lead to a stronger and longer-lasting effect than the vaccine alone. The companies are aiming to launch a Phase 1 trial in the second half of this year. ► April 8, 2020 The FDA issued an emergency use authorization for an antibody test for COVID-19. Developed by Cellex, the test looks for antibodies in the blood that the body produces against SARS-CoV-2 instead of the virus itself. Thiolanox (high-dose inhaled nitric oxide therapy) can now be tested in patients infected with SARS-CoV02 as part of a Phase 2 clinical trial (NCT03331445) that began in 2017 to assess its effects in treating difficult lung infections. Mallinckrodt and Novoteris, developers of Thialonax, announced on April 1 that their joint pilot trial was cleared by the Therapeutic Products Directorate of Health Canada. The trial will now be able to recruit participants infected with SARS-CoV-2 at Vancouver Coastal Health Authority facilities to test the therapy's safety and effectiveness in treating COVID-19. The FDA approved a randomized, double-blind, placebo-controlled Phase 3 trial (NCT04320615) to evaluate the safety and efficacy of Actemra (tocilizumab) plus standard of care in hospitalized adults with severe COVID-19 pneumonia. Actemra is an anti-rheumatoid medication by Genentech that is also being evaluated in a number of other trials as a potential treatment for COVID-19. A Phase 3 trial called RUXCOVID is awaiting FDA clearance to evaluate the safety and efficacy of ruxolitinib in COVID-19-associated cytokine storm. The medication was developed by Incyte and is sold under the brand-name Jakafi for polycythemia vera, myelofibrosis, and acute graft-versus-host disease. Two more trials will test ruxolitinib in treating severe acute respiratory syndrome (SARS) caused by COVID-19 (NCT04334044) and COVID-19 pneumonia (NCT04331665). The trials will take place in Mexico and in Canada respectively, but are not yet recruiting patients. Researchers are working on a new RNA-based immunotherapy to treat COVID-19. The potential treatment would be inhaled by patients and produce therapeutic antibodies in the lungs. Neurimmune and Ethris are hoping to start trials testing the potential treatment by the end of the year. Preclinical studies have begun in Australia testing potential vaccines against COVID-19. Run by Australia's Commonwealth Scientific and Industrial Research Organization, these studies are also testing the best way to administer the vaccine. The Regulatory Affairs Professionals Society has released a COVID-19 Therapeutics Tracker. The tracker is updated weekly with details about new treatment candidates for COVID-19. ► April 1, 2020 Several tests for diagnosing COVID-19 have now been granted emergency use authorization (EUA) from the FDA. As of March 31, the Advanced Medical Technology Association estimated that 17 tests had received EUAs, with more in the works. The same day, Bodysphere announced it was rolling out a test that returned results within two minutes, saying it was granted an EUA, but the FDA later refuted that claim. The test at this point has not received any FDA authorization. The FDA issued a warning last week that it has not authorized any test for people to use at home for COVID-19 and that people should be aware of such fraudulent tests. It also provided a list of fraudulent COVID-19 products including those that claim to prevent or treat the disease. The agency also announced the creation of an emergency program called the Coronavirus Treatment Acceleration Program to assist in the development of treatments for COVID-19. According to the FDA, as of March 31, it is reviewing 10 therapies already in trials and 15 others in preclinical stages. Sandoz's malaria treatment hydroxychloroquine sulfate and Bayer Pharmaceuticals' chloroquine phosphate have been granted an EUA by the FDA for the treatment of COVID-19. Both companies have donated supplies of the medications to the U.S. Department of Health and Human Services for use. Although clinical trials are needed to prove their efficacy in treating COVID-19, these therapies have shown some benefit in the lab and clinic, according to anecdotal evidence. Among the trials recently launched for COVID-19 are a global Phase 2/3 trial (NCT04315298) testing rheumatoid arthritis treatment Kevzara (sarilumab) and a U.K.-based Phase 2 trial testing antiviral SNG001, an inhaled formulation of interferon-beta-1a. The first patients have already been treated in both trials. Another Phase 2/3 trial (NCT04315298) testing Kevzara is recruiting an estimated 400 participants in the U.S. A Phase 2 trial, taking place in New York and Haifa, Israel, (NCT04311697) will test aviptadil for the treatment of COVID-19-associated acute respiratory distress syndrome. Johnson & Johnson announced plans to start a Phase 1 trial by September to test its vaccine candidate Ad26 SARS-CoV-2 against COVID-19. If the trial is successful, the first batch of the vaccine could be available in early 2021 for emergency use, according to the company. ► March 27, 2020 The CDC has developed a diagnostic panel for use by CDC-qualified laboratories in the U.S. and made available under an emergency use authorization (EUA) from the FDA Other new tests include Roche Diagnostics' cobas SARS-CoV-2 test, also granted an EUA, and the U.S. Department of Health and Human Services is funding the development of two other diagnostic tests that can detect the presence of SARS-CoV-2 within one hour. The National Institute of Allergy and Infectious Diseases is sponsoring a randomized, controlled Phase 2 trial in the U.S. to evaluate the safety and efficacy of the broad-spectrum anti-viral treatment remdesivir by Gilead Sciences to treat the disease. Gilead has also launched two global Phase 3 trials to evaluate remdesivir's safety and efficacy in adults with COVID-19. Other treatments being investigated for COVID-19 include a novel mRNA-1273 nanoparticle-encapsulated vaccine (NCT04283461), thalidomide (NCT04273581), sildenafil (NCT04304313), eculizumab (NCT04288713), recombinant human interferon-alpha 1 beta (NCT04293887), bevacizumab (NCT04305106), and antibodies from cured patients (NCT04264858), among others. Researchers are also looking at new synthetic biology approaches by using self-assembling nanoparticles coated with viral antigens that can precisely target SARS-CoV-2. This approach can potentially overcome some of the limitations of conventional vaccines such as short shelf-life and viral evolution. What is COVID-19?COVID-19, short for coronavirus disease 2019, is an infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 is a newly identified pathogen that has not previously been seen in humans and is highly contagious. Though it belongs to the same category of viruses as SARS coronavirus (SARS-CoV) and influenza viruses, SARS-CoV-2 is a different strain with its own characteristics. COVID-19 was first reported in Wuhan, China, in December 2019, and the outbreak has spread quickly across the world, prompting the World Health Organization (WHO) to declare COVID-19 a pandemic. How does COVID-19 spread?Because COVID-19 is a new virus, nobody has prior immunity to it, meaning the entire human population is prone to infection. COVID-19 is primarily spread via respiratory droplets when people cough or sneeze. Scientists have yet to understand how easily and sustainably the disease can spread among people. Based on available evidence, researchers do not think that airborne spread is a major transmission route. What are the symptoms of COVID-19?Common symptoms of COVID-19 begin two to 14 days after exposure. They include fever, tiredness, and dry cough. Other symptoms include sputum production, shortness of breath, sore throat, headache, myalgia (muscle pain) or arthralgia (joint pain), chills, vomiting, and nasal congestion. Less frequent symptoms include diarrhea, hemoptysis (coughing up blood from the respiratory tract), and conjunctival congestion. Most of these symptoms are usually mild, and about 80% of people who get the virus will typically recover without needing any special treatment. However, about 1 in 6 patients become seriously ill and develop breathing difficulties. What general preventive measures should people take?The following simple preventive measures can help minimize the spread of COVID-19:
What extra precautions should pulmonary hypertension patients take?Individuals with respiratory diseases such as pulmonary hypertension do not appear to be more at risk for contracting COVID-19 than the general public. However, they may be more likely to get a chest infection from the virus. Patients should, therefore, take extra precautions to minimize the risk of getting COVID-19. In addition to the general preventive measures listed above, they should:
Those with existing respiratory problems should only wear face masks when necessary as they can make breathing more difficult. If symptoms of a viral infection appear and patients have traveled to a high-risk area in the past 40 days, they should self-isolated at home for 14 days. They should maintain their daily care regimens and speak to their healthcare providers for any specific queries about their personal health. Most patients with respiratory diseases typically recover from COVID-19. Advice for family members and caregiversFamily members and caregivers of people with chronic diseases like pulmonary hypertension should take appropriate precautions and take extra care to avoid bringing COVID-19 home. They should constantly monitor patients and stock medicines and other necessary supplies that can last for several weeks. Storing extra non-perishable food can help minimize trips to the grocery store. People who show symptoms of COVID-19 should avoid visiting their family members in nursing homes or other places until the self-isolation period is complete. What should sick individuals do?If symptoms are present and a COVID-19 diagnosis is confirmed, patients should follow these steps to prevent the spread of the infection:
People should call ahead before visiting the hospital for an appointment. This way, the hospital can take necessary steps to prevent the spread of the infection. Patients who have confirmed COVID-19 should wear face masks when going out. Healthcare professionals and caregivers working with COVID-19 patients should also wear face masks. The CDC does not recommend that healthy people wear a face mask. The World Health Organization (WHO)'s website has a resource explaining the proper use of a face mask. What tests are available?Many tests for the detection of COVID-19 have been made available under the FDA's emergency use authorization, including rapid tests that are being developed to detect the presence of the virus within minutes. The Foundation for Innovative New Diagnostics provides an up-to-date list of different manual and automated tests that are available or currently in development. Is there a treatment?There are currently no vaccines available for human coronaviruses including COVID-19. This makes the prevention and containment of the virus very important. Oxygen therapy is the major treatment intervention for patients with severe disease. Mechanical ventilation may be necessary in cases of respiratory failure. Are there new treatments in the pipeline?Several clinical trials have been launched or are being planned to test a variety of potential treatments and vaccines for COVID-19. A complete list of all ongoing clinical trials pertaining to the virus is available here. *** Pulmonary Hypertension News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. |
| You are subscribed to email updates from "pulmonary hypertension causes" - Google News. To stop receiving these emails, you may unsubscribe now. | Email delivery powered by Google |
| Google, 1600 Amphitheatre Parkway, Mountain View, CA 94043, United States | |
Comments
Post a Comment