New hope against diseases marked by progressive scarring of lung tissue - Medical Xpress
New hope against diseases marked by progressive scarring of lung tissue - Medical Xpress |
- New hope against diseases marked by progressive scarring of lung tissue - Medical Xpress
- Pulmonary Hypertension Fund Opens at the HealthWell Foundation - PRNewswire
- Tracleer for Digital Ulcers Lowers PH Risk in Scleroderma, Study Finds - Scleroderma News
- Pulmonary Arterial Hypertension Epidemiology Forecast to 2030 by DelveInsight - Press Release - Digital Journal
| New hope against diseases marked by progressive scarring of lung tissue - Medical Xpress Posted: 15 Jan 2021 12:00 AM PST  (HealthDay)—An inhaled medication might make every day physical activity a bit easier for patients with serious scarring of the lungs, a new clinical trial finds. The study, published online Jan. 13 in the New England Journal of Medicine, involved patients with high blood pressure in the lungs caused by interstitial lung disease (ILD). ILD is a broad term for progressive scarring of the tissue surrounding the lungs' air sacs and blood vessels. It can have a range of causes, from smoking, to occupational exposure to toxins like asbestos, as well as autoimmune diseases like rheumatoid arthritis. Sometimes, no cause can be found. A potential, and serious, complication of that scarring is pulmonary hypertension, in which the vessels that supply blood to the lungs become hard and narrow. Once pulmonary hypertension arises, patients can become so short of breath they have difficulty walking, and often need to use more supplemental oxygen. The complication may also shorten their lives. Right now, no medication is approved specifically for pulmonary hypertension caused by ILD, said Dr. Steven Nathan, senior researcher on the new trial. He's director of the Advanced Lung Disease and Lung Transplant Program at Inova Fairfax Hospital in Falls Church, Va. There are, however, drugs for another form of pulmonary hypertension, known as pulmonary arterial hypertension. Those medications are vasodilators, which means they help blood vessels in the lungs relax and widen. And Nathan's team found that one of them—an inhaled medication called Tyvaso (treprostinil)—improved exercise capacity in patients with ILD. Over 16 weeks, patients randomly assigned to use the drug were able to improve their performance on a six-minute treadmill walk. Beyond that, their lung disease remained more stable. About 23% showed a worsening, compared to 33% of patients randomly assigned to take a placebo (an inactive inhaled solution). The trial is the first to "unequivocally" show benefits of vasodilator medication for patients with pulmonary hypertension caused by ILD, according to Nathan. The study period was short, however, (16 weeks) and it's unclear what the long-term effects would be, including whether the therapy can help extend patients' lives. But current medications for pulmonary arterial hypertension have been approved on the basis of even shorter trials, Nathan pointed out. He said the new findings should be enough for the U.S. Food and Drug Administration to approve Tyvaso for ILD patients. "It's enough evidence for me to prescribe it for my patients," Nathan added. (Doctors are allowed to prescribe FDA-approved medications for unapproved uses when they believe it's medically appropriate.) Dr. Albert Rizzo, chief medical officer of the American Lung Association, described the results as encouraging. "These patients are very impaired," he said, and an improvement on the six-minute walk test is important. In part, that's because walking is a key part of managing the lung disease. "We want patients to stay active," Rizzo said, "and walking is the best exercise for them." A broader goal, he said, is to improve patients' quality of life. In this trial, patients using Tyvaso did not rate their quality of life any higher than placebo patients did. But the study's primary goal was to assess exercise capacity, which is an initial step, Rizzo said. Larger, longer studies are needed to answer questions about quality of life and survival, he added. The trial, funded by Tyvaso maker United Therapeutics, involved 326 patients. Half were randomly assigned to use the inhaler drug every day for 16 weeks; the other half received an identical-looking placebo. During that time, patients on the drug improved their walking distance on treadmill tests, while placebo patients generally declined. On average, Tyvaso patients managed to go about 100-feet farther. The main side effects were cough and throat irritation from using the inhaler. If Tyvaso is approved for ILD patients, Nathan foresees it as being another tool doctors can prescribe, along with oxygen therapy and pulmonary rehabilitation (which involves supervised exercise). Even though doctors can prescribe the drug without an official approval, Rizzo said patients should be aware that their insurance plan might balk at covering it. And the drug is costly, topping $100,000 a year, according to CVS Health. Rizzo noted that many patients with serious, progressive lung disease may not know whether they have pulmonary hypertension. He suggested they ask their doctors whether they've been tested for it, and if they do have the condition, whether there are any additional therapies they should be getting. Explore further More information: The American Lung Association has more on interstitial lung disease. Copyright © 2020 HealthDay. All rights reserved. Citation: New hope against diseases marked by progressive scarring of lung tissue (2021, January 15) retrieved 25 January 2021 from https://medicalxpress.com/news/2021-01-diseases-scarring-lung-tissue.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only. |
| Pulmonary Hypertension Fund Opens at the HealthWell Foundation - PRNewswire Posted: 11 Jan 2021 06:26 AM PST GERMANTOWN, Md., Jan. 11, 2021 /PRNewswire/ -- The HealthWell Foundation®, an independent non-profit that provides a financial lifeline for inadequately insured Americans, is excited to announce that it has launched a new fund to assist individuals living with pulmonary hypertension (PH). Through the fund, HealthWell will provide up to $10,000 in medication copayment or insurance premium assistance for a 12-month grant period to eligible Medicare patients who have annual household incomes up to 500 percent of the federal poverty level. About Pulmonary Hypertension There are five groups of PH based on different causes as defined by the World Health Organization.
Regardless of the type, if left untreated, PH can result in right heart failure and death. There is no cure for PH, however, specific therapies are available to manage symptoms and improve quality of life. For additional information and resources, visit the Pulmonary Hypertension Association. "Prompt, accurate diagnosis and continuous treatment are essential for the pulmonary hypertension community. For people living with this potentially deadly disease, forgoing treatment is not an option," said Katie Kroner, Senior Director of Advocacy and Treatment Access for PHA. "Unfortunately, individuals living with PH often face significant financial barriers to care. Charitable assistance organizations like the HealthWell Foundation provide an essential safety net for those whose lives depend on these complex therapies. PHA is thrilled that a new resource is now available for PH community members struggling with premium and copay costs." "Symptoms of the disease can have a dramatic impact on physical activity, making day-to-day activities extremely tiring and difficult. For people living with PH, specialty care and having the financial resources to access that care, especially for patients on Medicare, can present overwhelming challenges," said Krista Zodet, HealthWell Foundation President. "PH is a disease that requires continued treatment. Any interruption in that treatment or going untreated can result in devastating consequences for the patient. We are proud that our dedicated donors recognize this critical need and that, thanks to their generosity, we are able to provide a financial lifeline to Medicare patients living with PH to enable them to access life-changing, potentially lifesaving, medical treatments without the added burden of figuring out how to pay for them." To determine eligibility and apply for financial assistance, visit HealthWell's Pulmonary Hypertension Fund page. To learn how you can support this or other HealthWell programs, visit HealthWellFoundation.org. About the HealthWell Foundation About the Pulmonary Hypertension Association CONTACT: SOURCE HealthWell Foundation  Related Links |
| Tracleer for Digital Ulcers Lowers PH Risk in Scleroderma, Study Finds - Scleroderma News Posted: 05 Jan 2021 12:00 AM PST People with scleroderma who were treated with Tracleer (bosentan) to prevent digital ulcers — open sores on the fingers or toes — saw their risk of developing pulmonary hypertension (PH) lowered almost four times, a study found. The study, "Effect of bosentan in pulmonary hypertension development in systemic sclerosis patients with digital ulcers," was published in the journal PLOS One. In scleroderma, poor blood flow and narrowed blood vessels can lead to ulcer formation (digital ulcers) on the toes and fingers, and particularly on the fingertips. It also causes the thickening of the connective tissue around the arteries of the lungs, which may result in pulmonary arterial hypertension or PAH — the most frequent cause of PH in scleroderma. Although several studies have investigated an association between digital ulcers and PAH, the results have been mixed, with some studies showing a link while others did not. Tracleer, a therapy developed by Actelion Pharmaceuticals (now part of Janssen), is approved in Europe to treat digital ulcers in both people with scleroderma and those with PAH. It works by stopping the action of endothelin, which is a molecule that causes blood vessels to narrow. However, whether patients treated with Tracleer for digital ulcers also have a lower risk of PAH is unclear. To find out, researchers at the Hospital Universitari de la Santa Creu i Sant Pau, in Spain, in collaboration with colleagues at the neighboring Hospital Universitari de Vall Hebron, analyzed the medical records of 222 people with SSc with a previous history of digital ulcers. A total of 59 patients (26.6%) had been treated with Tracleer for digital ulcers for at least one month, while 163 not given the medication were included as a control group. Of those selected, 201 (91%) were women, with a mean age of 63.9, including 138 patients with limited cutaneous scleroderma. An estimated pulmonary arterial pressure (mPAP) of more than 40 mmHg was observed in 21% of patients, thereby meeting the criterion for PH risk. For those given Tracleer, the median treatment length was 34 months, with the most common dose of 250 mg given twice daily to 60% of the participants. Tracleer is given as a tablet. Adverse events were reported in 35.6% of patients, including elevated liver enzymes and swelling; 27.1% of participants discontinued treatment. Most patients also were receiving other medications, known as vasodilators, to relax and widen blood vessels. During follow-up, 13.8% of patients treated with Tracleer and 23.7% of those without treatment developed PH, as estimated by echocardiogram, although this difference did not reach statistical significance. No major differences in blood flow parameters between treated and non-treated participants were observed. A statistical analysis found that participants without a history of Tracleer treatment had a 3.9 times higher risk of developing PH compared with treated patients. The risk in those who did not take a class of vasodilators called PDE5 inhibitors was 3.74 times higher. In patients who had never received vasodilators known as prostanoids, the risk was 2.65 times greater. No differences in risk were found among other concurrent treatments. After follow-up in the Tracleer group, lung function was stabilized without further decline (61.8% vs. 57% at study start) as measured by percentage carbon monoxide diffusing capacity (%DLCO) — a value to assess the lungs' ability to transfer oxygen to the blood. In contrast, the control group had significantly lower %DLCO values by the end of follow-up (65.5% vs. 60.5%). "In summary, our study demonstrates that patients who initiated bosentan [Tracleer] to prevent [digital ulcers] have a lower risk to present PH estimated by echocardiography and stabilizes DLCO," the researchers wrote. "A randomized control trial is warranted to demonstrate a protective effect of these specific drugs." Steve holds a PhD in Biochemistry from the Faculty of Medicine at the University of Toronto, Canada. He worked as a medical scientist for 18 years, within both industry and academia, where his research focused on the discovery of new medicines to treat inflammatory disorders and infectious diseases. Steve recently stepped away from the lab and into science communications, where he's helping make medical science information more accessible for everyone. × Steve holds a PhD in Biochemistry from the Faculty of Medicine at the University of Toronto, Canada. He worked as a medical scientist for 18 years, within both industry and academia, where his research focused on the discovery of new medicines to treat inflammatory disorders and infectious diseases. Steve recently stepped away from the lab and into science communications, where he's helping make medical science information more accessible for everyone. Latest Posts |
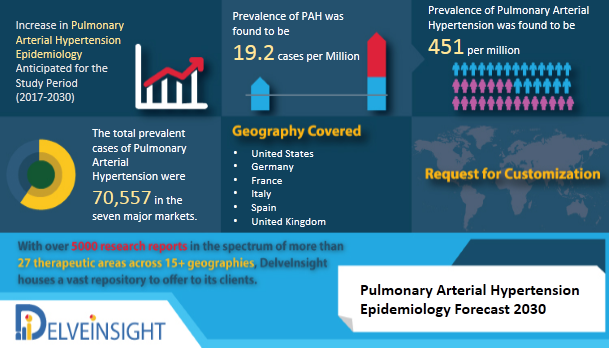
| Posted: 21 Jan 2021 02:11 PM PST DelveInsight has launched a new report on Pulmonary Arterial Hypertension Epidemiology Pulmonary Arterial Hypertension is a rare, chronic, and progressive form of Pulmonary Hypertension which is characterized by the elevated pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR) in blood vessels carrying the blood from the right side of the heart through lungs. It occurs due to the tightening and stiffening of the small pulmonary arteries leading to the right ventricular dysfunction and vessel obstruction. DelveInsight's 'Pulmonary Arterial Hypertension Epidemiology Forecast to 2030' report delivers an in-depth understanding of the disease, historical & forecasted epidemiology of Pulmonary Arterial Hypertension in the United States, EU5 (Germany, Spain, Italy, France and United Kingdom) and Japan. Pulmonary Arterial Hypertension Epidemiology The disease is found to be more common among women as compared to men. It is also observed that the patients with Systemic Sclerosis, Sickle Cell Disease, and Human Immunodeficiency Virus infection (HIV infection) are at a higher risk of developing Pulmonary Arterial Hypertension (PAH). Key facts of the Pulmonary Arterial Hypertension Epidemiology
Pulmonary Arterial Hypertension Epidemiology Segmentation
Pulmonary Arterial Hypertension Epidemiology Report Scope
• The report provides the insight about the historical and forecasted patient pool of Pulmonary Arterial Hypertension in 7 major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK) and Japan. • The Report assesses the disease risk and burden and highlights the unmet needs of the disease • The Report helps to recognize the growth opportunities in the 7MM with respect to the patient population • The report provides the segmentation of the disease total prevalent population of PAH, subtype-specific population of PAH and gender-specific PAH diagnosed pool in 7MM Request for free sample copy- https://www.delveinsight.com/sample-request/pulmonary-arterial-hypertension-epidemiology-forecast Table of content 1. Key Insights 2. Executive Summary of Pulmonary Arterial Hypertension 3. SWOT Analysis for Pulmonary Arterial Hypertension 4. Pulmonary Arterial Hypertension (PAH) Epidemiology Overview at a Glance 5. Disease Background and Overview: Pulmonary Arterial Hypertension (PAH) 6. Epidemiology and Patient Population 7. Country Wise-Epidemiology of Pulmonary Arterial Hypertension (PAH) 8. Treatment Algorithm 9. Unmet Needs 10. Case Studies 11. Organizations related with PAH 12. Appendix 13. DelveInsight Capabilities 14. Disclaimer 15. About DelveInsight
Request for free sample copy- https://www.delveinsight.com/sample-request/pulmonary-arterial-hypertension-epidemiology-forecast
Related Reports Media Contact |
| You are subscribed to email updates from "pulmonary hypertension causes" - Google News. To stop receiving these emails, you may unsubscribe now. | Email delivery powered by Google |
| Google, 1600 Amphitheatre Parkway, Mountain View, CA 94043, United States | |
Comments
Post a Comment