An organ-on-chip model of pulmonary arterial hypertension identifies a BMPR2-SOX17-prostacyclin signalling axis | Communications Biology - Nature.com
Abstract
Pulmonary arterial hypertension (PAH) is an unmet clinical need. The lack of models of human disease is a key obstacle to drug development. We present a biomimetic model of pulmonary arterial endothelial-smooth muscle cell interactions in PAH, combining natural and induced bone morphogenetic protein receptor 2 (BMPR2) dysfunction with hypoxia to induce smooth muscle activation and proliferation, which is responsive to drug treatment. BMPR2- and oxygenation-specific changes in endothelial and smooth muscle gene expression, consistent with observations made in genomic and biochemical studies of PAH, enable insights into underlying disease pathways and mechanisms of drug response. The model captures key changes in the pulmonary endothelial phenotype that are essential for the induction of SMC remodelling, including a BMPR2-SOX17-prostacyclin signalling axis and offers an easily accessible approach for researchers to study pulmonary vascular remodelling and advance drug development in PAH.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease of the pulmonary circulation characterised by the narrowing of pulmonary arteries and arterioles, leading to right ventricular hypertrophy and heart failure1. The disease is thought to be triggered by endothelial damage due to a combination of two or more hits, such as genetic alterations, hypoxia and inflammation, followed by exaggerated repair involving increased proliferation and resistance to apoptosis of cells in the arterial intimal and medial layers. In addition to this, endothelial-to-mesenchymal transition (EMT), aerobic glycolysis and conversion of the differentiated, contractile phenotype of smooth muscle cells to a proliferative, synthetic phenotype, are key contributors to vascular remodelling2. Existing drugs bring some symptomatic relief but, in most patients, do not arrest or reverse the disease3.
Rare genetic mutations have been identified that increase susceptibility to PAH4. A key genetic change is a heterozygous germline mutation in the gene encoding the bone morphogenetic protein type II receptor (BMPR2). Studies report BMPR2 mutations in over 80% of cases of familial PAH, and around 20% of sporadic or idiopathic PAH patients5. Non-genetic forms of PAH show reduced expression and increased degradation of the receptor6. While mutations in BMPR2 and other TGF-β receptor superfamily member genes account for most cases of heritable PAH, other genes and genetic loci have also been linked with the condition5,7.
Animal models do not fully reproduce the features of human PAH, which is a key obstacle to drug development8,9. Organs-on-chips have emerged in the last decade as a technology with huge potential to revolutionise in vitro disease modelling and increase the accuracy and throughput of pharmacological and toxicological screening. In addition, there is the potential to personalise medicine development through the use of patient-derived induced pluripotent stem cells and endothelial colony-forming cells (ECFCs).
Here, we present a microfluidic model of human pulmonary arterial endothelial–smooth muscle cell interactions cultured under the conditions of BMPR2 knockdown and hypoxia, the two known triggers of PAH. In this manuscript, this model is referred to as a two-hit model of PAH. We characterise functional and transcriptomic changes in these cells and validate the findings with the use of cells from PAH patients and mice with disabling BMPR2 mutations and comparative analysis of transcriptomic data from human PAH. The study identifies a BMPR2–SOX17–prostacyclin signalling axis, essential for the regulation of pulmonary arterial smooth muscle cell proliferation and demonstrates therapeutic effects of the endothelin receptor antagonist Ambrisentan10, a tyrosine kinase inhibitor Imatinib11, and AZD5153, a novel inhibitor of bromodomain-containing protein 4 (BRD4)12.
Results
Pulmonary artery-on-a-chip reconstitutes features of human lung arteries
The pulmonary artery-on-a-chip (PA-on-a-chip) was designed to monitor the responses of human pulmonary vascular endothelial and smooth muscle cells to pathological and potential therapeutic interventions. The polydimethylsiloxane (PDMS)-based device comprises two microfluidic channels (200 µm height × 1000 µm width) separated by a nanoporous polyethylene terephthalate (PET) membrane, with human pulmonary artery endothelial cells (HPAECs) and human pulmonary artery smooth muscle cells (HPASMCs) cultured on either side of the membrane (Fig. 1a, b). Membrane porosity (pore size 400 nm, density 2 × 106 pores/cm2) enables direct communication between cells, reflective of the role of the basal lamina in pulmonary arteries13,14. Inlets and outlets of endothelial channels are connected with tubing and pulse dampeners, to allow circulation of culture medium, actuated by laminar shear stress under physiological flow rates of 6 dynes/cm2, characteristic of human lung arterioles15 (Fig. 1c, d and Supplementary Figs. 1–3). The basic principles of the design were derived from a lung-on-a-chip model16.
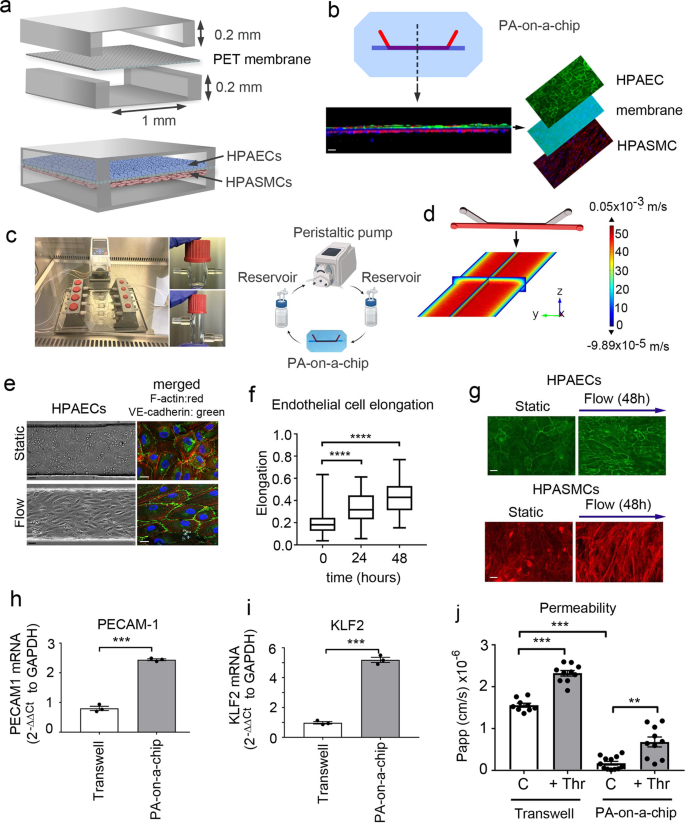
a Schematic diagram of the pulmonary artery-on-a-chip. b Z-section of endothelial-smooth muscle interface, fluorescent confocal imaging. HPAECs (green: VE-cadherin), HPASMCs (red: α-smooth muscle actin). Bar = 10 µm. c An image and a schematic diagram of the flow system set up comprising a peristaltic pump, media reservoirs and chips, perfused with culture medium at 6 dynes/cm2. d Fluid–structure interaction (velocity profile) in the endothelial channel, COMSOL modelling. e Phase contract (left) and fluorescent microscopy (right) images of HPAECs grown in microfluidic channels under static and flow conditions, as indicated. F-actin: red, VE-cadherin: green. Bar = 10 µm. f Endothelial cell elongation; n = 4 individual chips per time point. Error bars indicate mean ± SEM of a one-way ANOVA with a Tukey's post-hoc correction test. ****P < 0.0001. g HPAEC and HPASMC phenotype under static conditions and underflow (48 h) in PA-on-a-chip; fluorescent microscopy, F-actin (red) and VE-cadherin (green); Bar = 10 µm. h, i mRNA expression of endothelial differentiation markers, PECAM-1 and KLF2 in cells treated, as indicated; qPCR. ***P < 0.001; Unpaired t-test. n = 3. j Effects of thrombin (1 U/mL) on endothelial barrier function in HPAECs co-cultured with HPASMCs in pulmonary artery-on-a-chip or in transwell dishes. Passage of FITC-dextran (1 mg/mL; 1 h) from top to bottom channel was used as a measure of endothelial permeability. Cells from three different biological donors grown 3–4 chips/transwells per treatment group, were used; n = 10–12. Error bars indicate mean ± SEM of a one-way ANOVA with a Tukey's post-hoc correction test. **P < 0.01; ****P < 0.0001, comparisons, as indicated.
The alignment of HPAECs and HPASMCs within the device was reminiscent of the arterial intimal and medial cell configurations in vivo (Fig. 1e–g and Supplementary Fig. 4). Flow-stimulated HPAECs showed enhanced junctional localisation of vascular endothelial (VE)-cadherin and significantly elevated expression of endothelial maturity markers PECAM-1 and KLF2 (Fig. 1h, i). Endothelial barrier functionality was evaluated by measuring the passage of fluorescently-labelled 40 kDa dextran, which has a Stokes radius similar to human plasma albumin17, across the endothelial and smooth muscle layers stimulated with thrombin, a known vasoconstrictor and permeability factor18. HPAECs in the PA-on-a-chip showed enhanced endothelial barrier function and a larger increase in thrombin-induced permeability over baseline, compared with unstimulated controls (3.9-fold increase in PA-on-a-chip vs 1.5-fold increase in static culture) (Fig. 1j). HPASMCs co-culture did not affect endothelial barrier function under the study conditions (Supplementary Fig. 5).
Establishment of a PAH model on-a-chip
Remodelled PAH vasculature is characterised by intimal and medial thickening (Fig. 2a, b). There is a significant need to understand how endothelial BMPR2 haploinsufficiency, in combination with the second hit created by hypoxia or inflammation, elicits disease.
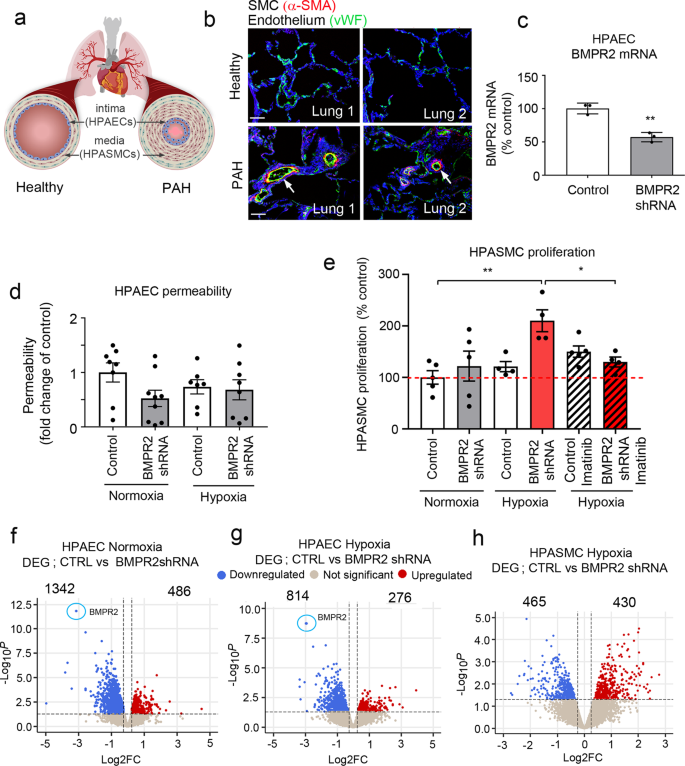
a Schematic diagram of pulmonary vascular remodelling in PAH. b α-SMA (red) and vWF (green) staining in healthy and PAH lungs. Bar = 50 µm. c BMPR2 mRNA expression in HPAECs treated AdCTRLshRNA with Ad-BMPR2-shRNA-GFP. n = 3; **P < 0.01; unpaired t-test. d Effect of two-hit (hypoxia and BMPR2 knockdown, 24 h) on HPAEC permeability, measured as the passage of 40 kDa FITC-dextran (1 mg/mL) from top to bottom channel; n = 9 individual chips. e Effect of hypoxia and endothelial BMPR2 knockdown on HPASMC proliferation (24 h, EdU assay). Cells were untreated or treated with 10 µM Imatinib mesylate for 24 h. n = 4–5 individual chips. In d, e error bars are mean ± SEM; one-way ANOVA with Tukey post-hoc correction test. Volcano plots show differentially expressed genes (DEG) in f HPAECs treated with BMPR2shRNA, g HPAECs in the two-hit PAH model (BMPR2 shRNA and hypoxia), h HPASMCs in the two-hit PAH model. Downregulated genes are in blue and upregulated genes are in red; P < 0.05, 0.25-fold cut-off. n = 4.
To recreate disease conditions in the PA-on-a-chip, HPAECs infected with AdBMPR2 shRNA were exposed to hypoxia (2% O2) for 24 h. The adenoviral infection was optimised to achieve ~50% reduction in BMPR2 expression, reflective of BMPR2 haploinsufficiency in PAH (Fig. 2c).
BMPR2 knockdown and hypoxia, separately or in combination, did not affect endothelial permeability or proliferation in PA-on-a-chip (Fig. 2d and Supplementary Fig. 6a), in contrast with prior observations in static cell culture19. HPASMC proliferation was significantly increased under the two-hit conditions when BMPR2 knockdown and hypoxia were combined (P < 0.01; Fig. 2e). This response was inhibited by imatinib mesylate, a receptor tyrosine kinase inhibitor currently under investigation as an anti-proliferation treatment of PAH (P < 0.05; Fig. 2e). No significant loss of cells from endothelial or smooth muscle channels was noted and none of the treatments affected cell apoptosis (Supplementary Figs. 6b, c and 7).
Genotype- and oxygenation-specific transcriptomic signatures from the two-hit microfluidic model of PAH
Control and BMPR2-deficient HPAECs co-cultured with HPASMCs under normoxic or hypoxic conditions underwent RNAseq analysis to identify key transcriptomic changes induced under the two-hit conditions. BMPR2 knockdown alone induced differential expression of 1828 genes in HPAECs (|logFC | >0.25, P < 0.05, comparison with controls), where, as expected, reduction in BMPR2 expression was the most significant change (−2.9-fold change, p = 2.07 × 10−9) (Fig. 2f). These genes showed enrichment in epithelial-to-mesenchymal transition (EMT), G2M checkpoint, myc targets, cell cycle (E2F targets) and DNA repair pathways (Hallmark, FDR < 0.01). HPASMCs co-cultured with these cells showed differential expression of 751 genes (Supplementary Fig. 8), associated predominantly with hypoxia, glycolysis, EMT and cholesterol homoeostasis (Hallmark, FDR < 0.01).
The combination of BMPR2 knockdown with hypoxia-induced differential expression of 1090 genes in HPAECs and 895 genes in HPASMCs. The pattern of these changes is illustrated in volcano plots (Fig. 2g, h) and heatmaps showing distinct hypoxia-, genotype- and two-hit-related gene clustering (Supplementary Fig. 9). A list of differentially expressed genes (DEG) in HPAECs and HPASMCs in the two-hit model of PAH is provided in Supplementary Data 1.
Transcriptional profiling of HPAECs from the two-hit model identified multiple genes linked with known PAH pathways, including TGF-β signalling, NOS pathway, proliferation, angiogenesis, Notch signalling, EndoMT, ion channels, fibrosis, and inflammation (Fig. 3a and Supplementary Fig. 10a). ~80% of the observed changes could be attributed to BMPR2 knockdown, including the downregulation of genes in TGF-β, NOS signalling pathway, junctional proteins and growth factor signalling mediators. DEG induced by hypoxia in BMPR2-deficient HPAECs and the corresponding GSEA pathway analysis data is provided in Supplementary Data 2 and 3.
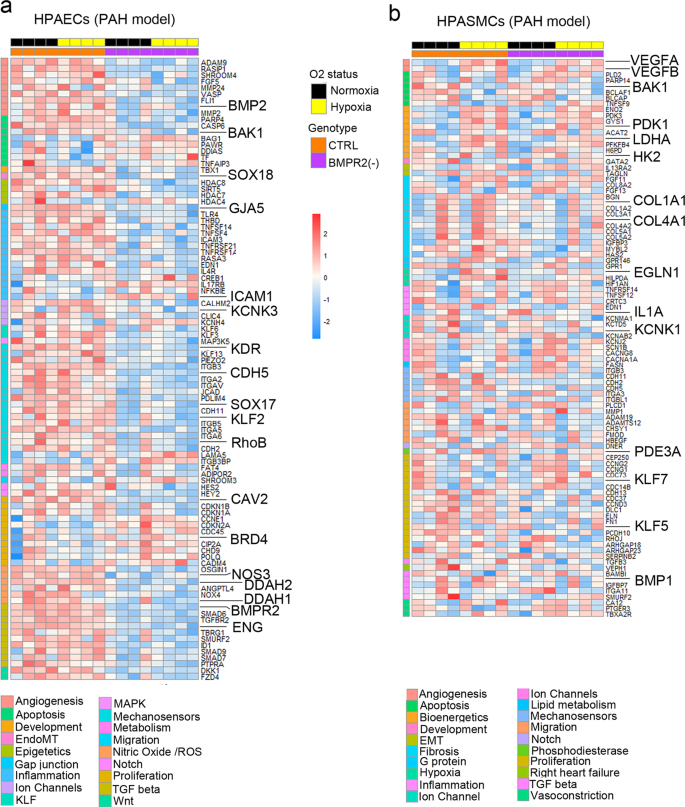
Top DEG associated with cardiovascular diseases were grouped into categories and visualised as transcripts per million (TPM) heatmaps. Colour-coded pathways are shown underneath the heatmaps, with key gene symbols enlarged in a HPAECs and b HPASMCs. The genotype status (control and BMPR2 knockdown) and oxygen status (normoxia or hypoxia) are shown at the top of the heatmap in different colours, as indicated. Changes in gene expression are also colour coded, with blue denoting a lower relative gene expression and red denoting a higher relative gene expression. Each column represents 1 experimental repeat (n = 4/treatment group).
HPASMCs in the two-hit PAH model showed associations with several pathways involved in vascular remodelling, including angiogenesis, apoptosis, inflammation, vasoconstriction and TGF-β signalling (Fig. 3b, Supplementary Fig. 10b). Only ~30% of these changes could be attributed to the endothelial BMPR2 knockdown. The additional genes affected by hypoxic exposure were linked to glycolysis, adipogenesis, TNF-α, cell cycle, Rho signalling, and ECM interactions. DEG affected by HPAEC BMPR2 silencing in normoxic HPAECs and HPASMCs are provided in Supplementary Data 4. Hypoxia-regulated HPASMC genes in the two-hit model and the corresponding GSEA pathway analysis data are provided in Supplementary Data 2 and 5, respectively.
Changes in the expression of selected HPAEC and HPASMC gene targets were validated by qPCR and immunofluorescent analysis (Supplementary Figs. 11 and 12). To further validate our findings, we measured the activity of hexokinase, the rate-limiting enzyme in the glycolysis and pentose cycle, in HPASMCs. Hexokinase activity in HPASMCs cultured under basal, control conditions was 0.86 ± 0.043 nM NADH/min/mL. It increased under hypoxic conditions (3.4 ± 0.9-fold increase, P < 0.05, comparison with controls) and was further markedly elevated in HPASMCs cultured with HPAECs under the two-hit conditions (6.3 ± 0.4-fold increase, P < 0.0001, comparison with controls) (Supplementary Fig. 13).
Model validation with PAH cells with BMPR2 mutations
Blood-derived endothelial colony-forming cells (ECFCs) are often used as surrogates for pulmonary endothelial cells in PAH and display abnormalities in key pathways linked to the disease pathogenesis20,21.
To investigate whether the effects seen in the two-hit model of PAH would yield similar findings in patient-derived cells, we opted to substitute HPAECs with ECFCs from PAH patients with disabling BMPR2 mutations. The process of isolation and culture of ECFCs is illustrated in Fig. 4a. PAH ECFCs did not show major morphological differences with healthy controls (Fig. 4b, c and Supplementary Fig. 14). However, consistent with the responses seen in the two-hit PAH model, HPASMCs co-cultured with PAH ECFCs under hypoxic conditions showed a significant ~2-fold increase in cell proliferation (Fig. 4d). No changes in ECFC and HPASMC apoptosis were observed (Supplementary Fig. 15).
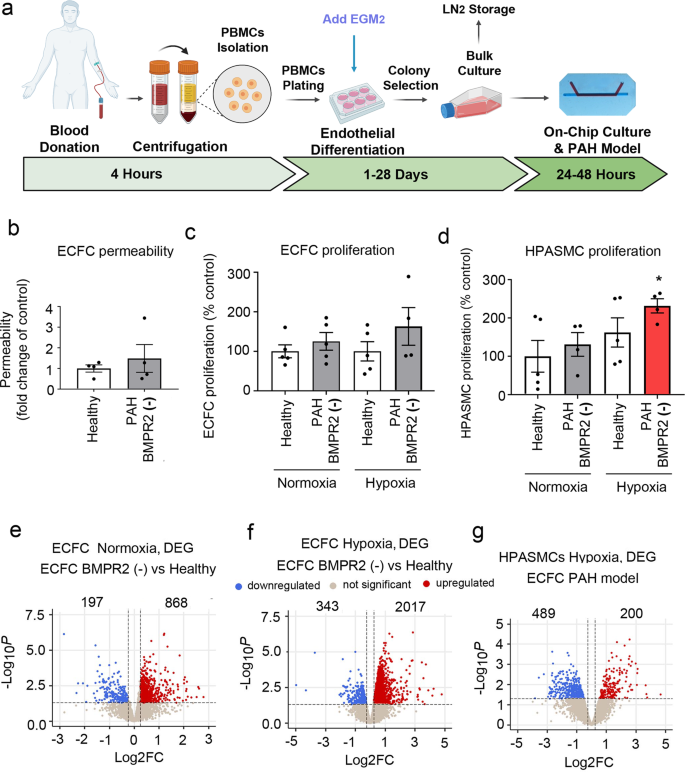
a Schematic diagram of isolation and culture of patient ECFCs. b Permeability of ECFCs from healthy individuals and PAH patients with BMPR2 mutations cultured in PA-on-a-chip. c Proliferation of healthy and PAH ECFCs in PA-on-a-chip under normoxic or hypoxic conditions (2% O2, 24 h), as indicated. d Proliferation of HPASMCs co-cultured with control or patient ECFCs under normoxic or hypoxic conditions. n = 4–5 biological donors, each assayed in a separate chip. Bars are means ± SEM; one-way ANOVA with a Tukey post-test; *p ≤ 0.05. Volcano plots show e DEG in PAH ECFCs with BMPR2 mutations vs. healthy controls in normoxia, f DEG in PAH ECFCs vs. healthy controls in hypoxia (ECFC PAH model), g DEG in HPASMCs in hypoxia. In e–g n = 5 biological donors, each in a separate chip.
Normoxic PAH ECFCs with BMPR2 mutations showed 1065 differentially expressed genes (DEG) versus healthy controls (Fig. 4e), associated predominantly with ROS, KRAS, mTOR signalling and adipogenesis (Hallmark, p < 0.01). 15% of this gene pool was shared with BMPR2-deficient HPAECs and included key regulators of vascular homoeostasis such as DDAH1, CAV1, PDGFB, KLF2, APLN (Supplementary Data 6). HPASMCS co-cultured with PAH ECFCs showed 972 DEG (Supplementary Fig. 16) enriched in cell cycle progression (E2F targets), G2M checkpoint, oxidative phosphorylation, interferon-gamma pathways (Hallmark, FDR < 0.01). A list of DEG in BMPR2-deficient ECFCs and HPASMCs co-cultured with these cells under normoxic conditions is provided in Supplementary Data 7.
PAH ECFCs from the two-hit ECFC PAH model (combining PAH BMPR2 mutations and hypoxia) showed 2360 DEG, while HPASMCs showed 689 DEG (logFC > 0.25, P < 0.05) (Fig. 4f, g and Supplementary Data 1). Hierarchical gene clustering in ECFCs and HPASMCs under different experimental conditions revealed genotype- and oxygenation-specific changes in gene expression (Fig. 5a, b and Supplementary Fig. 17).
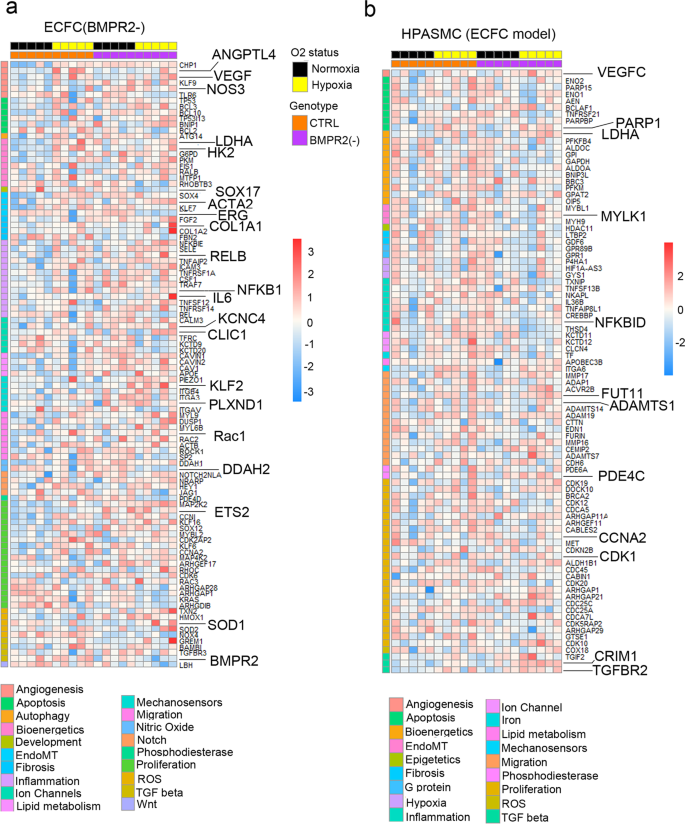
Top DEG associated with cardiovascular diseases were grouped into categories and visualised as TPM heatmaps. Colour-coded pathways are shown underneath the heatmaps, with key gene symbols enlarged in a PAH ECFCs and b HPASMCs co-cultured with ECFCs. The genotype status (control and BMPR2 knockdown) and oxygen status (normoxia or hypoxia) are shown at the top of the heatmap in different colours, as indicated. Changes in gene expression are also colour coded, with blue denoting a lower relative gene expression and red denoting a higher relative gene expression. Each column represents 1 donor; n = 5 different biological donors/treatment groups.
ECFC DEG from the two-hit model showed enrichment in key PAH pathways, including angiogenesis, apoptosis, EMT, fibrosis, inflammation, proliferation, nitric oxide, TGF-β, Notch and Wnt signalling (Hallmark, FDR < 0.01) (Fig. 5a and Supplementary Fig. 18a). Only ~30% of these genes were related to BMPR2 mutation. The remaining ~70% of genes induced by additional hypoxic exposure showed links with glycolysis, adipogenesis, cell cycle, oxidative phosphorylation, Rho signalling, TNF-α signalling and cellular senescence (Supplementary Data 8).
HPASMC genes from the two-hit ECFC PAH model showed significant associations with angiogenesis, apoptosis, hypoxia, inflammation, proliferation, fibrosis and TGF-β pathways (Fig. 5b and Supplementary Fig. 18b). 38% of these genes could be related to the BMPR2 loss of function, with 62% of changes affected by hypoxia and linked with glycolysis, pentose metabolism, cell cycle, and immune responses. A list of HPASMC genes affected by ECFC BMPR2 mutations is provided in Supplementary Data 6. Hypoxia-regulated genes in these cells and corresponding GSEA pathway analysis data are shown in Supplementary Data 2 and 9, respectively.
Transcriptomic overlaps between different PAH datasets
RNAseq datasets from the two microfluidic models of endothelial dysfunction in PAH were compared with published gene datasets from IPAH lung transplants22,23 and other gene databases with known PAH associations24. DEG from our models and other published datasets are listed in Supplementary Data 10 and 11. The results of the comparative analysis are shown in Fig. 6.
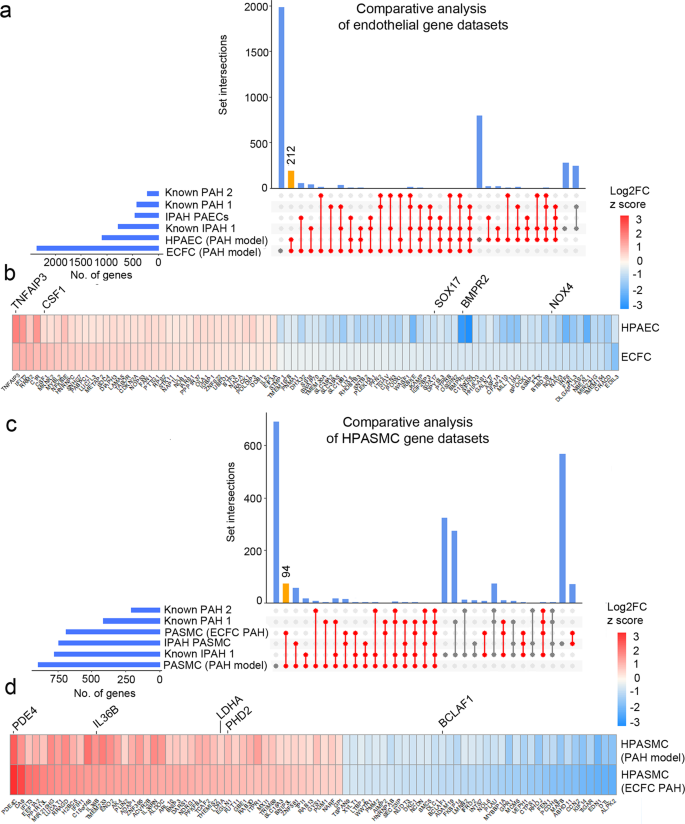
DEG datasets from the adenoviral two-hit models (PAH Model) and patient ECFC models (ECFC PAH) of PAH were compared against previously reported RNAseq studies and lists of genes known to be associated with PAH and IPAH. UpSet plots and heatmaps detail comparative analysis of a, b endothelial gene datasets and c, d smooth muscle cell datasets. In a, c the largest gene overlaps are highlighted in yellow. Heatmaps in b, d visualise the similarity of gene expression changes between the two microfluidic models of PAH, the two-hit PAH model and the ECFC PAH model; downregulated genes are in blue and upregulated genes are in red. RNAseq datasets used for comparative analysis were from PASMCs from IPAH lung transplants22 (database named here IPAH PASMCs); PAECs isolated from IPAH lung transplants23 (named here IPAH PAEC). Other comparative gene sets included genes with known PAH-associations 24 (here named "known PAH2") and the DisGENET public database https://www.disgenet.org/search, where following gene sets were obtained: DisGENET PAH (https://www.disgenet.org/browser/0/1/0/C2973725/), (here named "known PAH1"); DisGENET IPAH (https://www.disgenet.org/browser/0/1/0/C3203102/) (here named "known IPAH1").
The highest number of shared endothelial genes was found between our two microfluidic PAH models (212 genes), between the microfluidic ECFC PAH model and the IPAH PAEC gene dataset (63 genes) and between the two-hit PAH model and the IPAH PAEC dataset (33 genes)24 (Fig. 6a and Supplementary Data 11). GO pathway analysis (Hallmark) of the overlapping endothelial gene datasets revealed their associations with key PAH pathways, including EMT, TNF-α signalling via NFkB, hypoxia, apoptosis, and p53 pathway (FDR < 0.05) (Supplementary Data 12).
Consistently, the highest number of shared differentially expressed SMC genes was found between our microfluidic PAH models (94 genes), between the two-hit ECFC PAH model and IPAH PASMCs25 (86 genes) and between the two-hit HPAEC PAH model and IPAH PASMCs (76 genes) (Fig. 6c and Supplementary Data 10). The shared SMC gene set showed links with TGF-β signalling, glycolysis, fatty acid metabolism, pentose phosphate pathway, TNF-α via NFκB signalling, cell cycle/G2M checkpoint (GO pathway analysis, FDR < 0.05) (Supplementary Data 13).
Endothelial SOX17 regulates PASMC proliferation
Loss of endothelial SOX17 was one of the most prominent changes seen in BMPR2-deficient cells in microfluidic models of PAH and PAH databases. Consistently, expression of SOX17 was markedly reduced in the lung endothelium of mice carrying a disabling ligand-binding domain mutation of BMPR2 (BMPR2C118W)26 (Fig. 7a–c and Supplementary Fig. 19). Reduced nuclear localisation of SOX17 was also noted in hypoxic, BMPR2-deficient HPAECs (Fig. 7d and Supplementary Fig. 20).
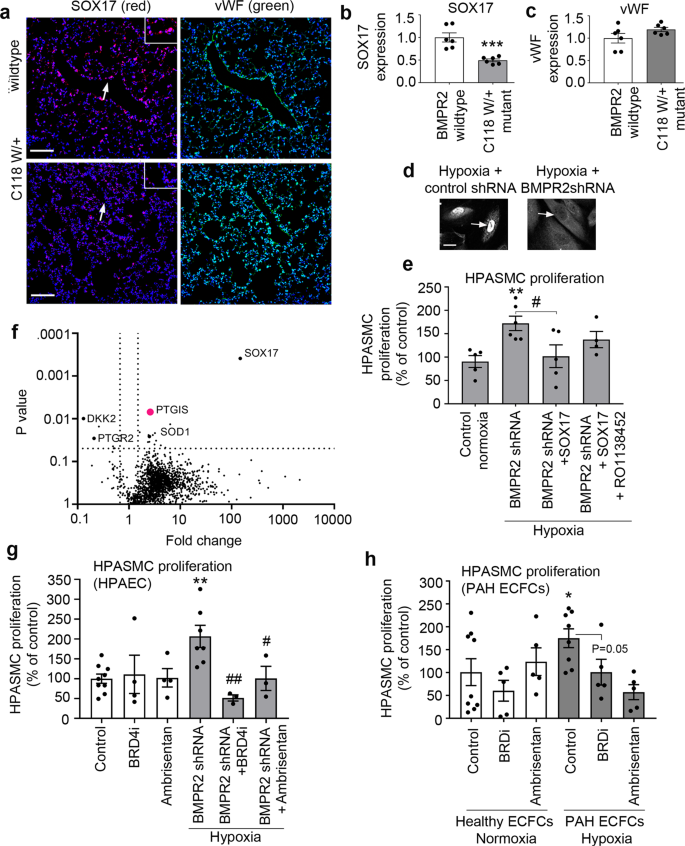
a Representative confocal images of lung sections of wildtype and BMPR2 (C118W/+) mice with SOX17 (red) and vWF marking endothelium (green) and nuclei in blue (DAPI), as indicated; Bar = 50 µm. Arrows point to cell nuclei. Magnified images of boxed areas are shown in the top right corner. b, c Corresponding graphs showing SOX17 and vWF expression in mouse lung tissues, as indicated. d Confocal images showing localisation of SOX17 in BMPR2-deficient HPAECs (BMPR2 shRNA) and controls (control shRNA), as indicated. Arrows point to cell nuclei. Bar = 10 µm. e Volcano plot showing SOX17-induced DE proteins, with prostacyclin synthase (PTGIS) marked in pink and selected proteins of interest highlighted. f Effect of endothelial SOX17 overexpression on HPASMC proliferation in the two-hit model (BMPR2shRNA + hypoxia) model, with and without prostacyclin receptor inhibitor, RO1138452 (10 µM). g, h Effect of Ambrisentan and BRD4 inhibitor AZD5153 on HPASMC proliferation in two-hit models of PAH, utilising BMPR2-deficient HPAECs or PAH ECFCs, as indicated. In b, c n = 6/group. ***P < 0.0001, Student t-test; In e n = 4–6, *P < 0.05 comparison with normoxic control, #P < 0.05, comparison as indicated; one-way ANOVA with Tukey post-test. In g n = 4–8, in h n = 5–10.
To investigate the potential role of endothelial SOX17 in the regulation of PASMC proliferation, a compensatory overexpression of SOX17 was induced in BMPR2-deficient HPAECs. SOX17 prevented an increase in PASMCs proliferation under the two-hit conditions (Fig. 7e). Quantitative proteomic analysis of SOX17-overexpressing HPAECs identified 20 potential mediators of this response, including prostacyclin synthase (PTGIS), prostaglandin reductase (PTGR2), superoxide dismutase 1 (SOD1), Dickkopf WNT Signalling Pathway Inhibitor 2 (DKK2) and Krüppel-like factor 16 (KLF16). An increase in prostacyclin synthase expression was the most significant change noted (2,5-fold increase, P = 0.006) (Fig. 7f). Normalised and raw protein abundances in study groups are provided in Supplementary Data 14–16.
Selective prostacyclin receptor inhibitor RO113845227 abolished the anti-proliferative actions of SOX17, reaffirming the key role of prostacyclin in the regulation of PASMC proliferation under the two-hit disease conditions.
Prostacyclin exerts vasodilatory and anti-proliferative effects on vascular SMCs and its deficiency has been linked to the reduced expression of PTGIS in PAH28,29. We identified eight predicted SOX17-binding sites within the PTGIS promoter and eleven binding sites within its putative enhancer regions, suggestive of direct regulatory interaction (Supplementary Fig. 21). Multiple SOX17-binding sites were also found in promoters and enhancers of other PAH-relevant genes, including PTGIS, PTGR2, DKK2, except for SOD1, which included only one predicted SOX17-binding site in one of its enhancers.
Drug testing in microfluidic two-hit models of PAH
To test if PASMC proliferation induced under the two-hit conditions is responsive to treatment with PAH-relevant drugs, Ambrisentan, an FDA-approved selective antagonist of ET-1 receptor A and AZD5153, a compound from a structural class of BET inhibitors, were added to the flow system. Two BRD-binding motifs (warheads) of AZD515329 enable bivalent-binding and confer high potency for BRD4, differentiating this drug from alternatives including Apabetalone (RVX-208)12. At clinically relevant10,12 concentrations both Ambrisentan and AZD5153 effectively inhibited PASMC proliferation (Fig. 7g, h).
Discussion
We present an organ-on-chip model of vascular endothelial and smooth muscle cell interactions that permits the study of dynamic changes in molecular and functional cell phenotype under physiological flow conditions in response to key factors linked to the development of PAH, such as BMPR2 silencing and hypoxia. The study describes pulmonary endothelial cell phenotype changes required for the induction of medial smooth muscle activation and proliferation and highlights the potential importance of a link between BMPR2 and an arterial identity determinant, SOX17 in the regulation of this response. The model can accommodate blood-derived endothelial cells from patients, offering the prospect of tailoring medicines to individual patients. PASMC proliferation, a key feature of medial hyperplasia, was attenuated by five drugs exhibiting different modes of action, validating the use of this platform for drug testing. The experimental design and key findings are summarised in Fig. 8.
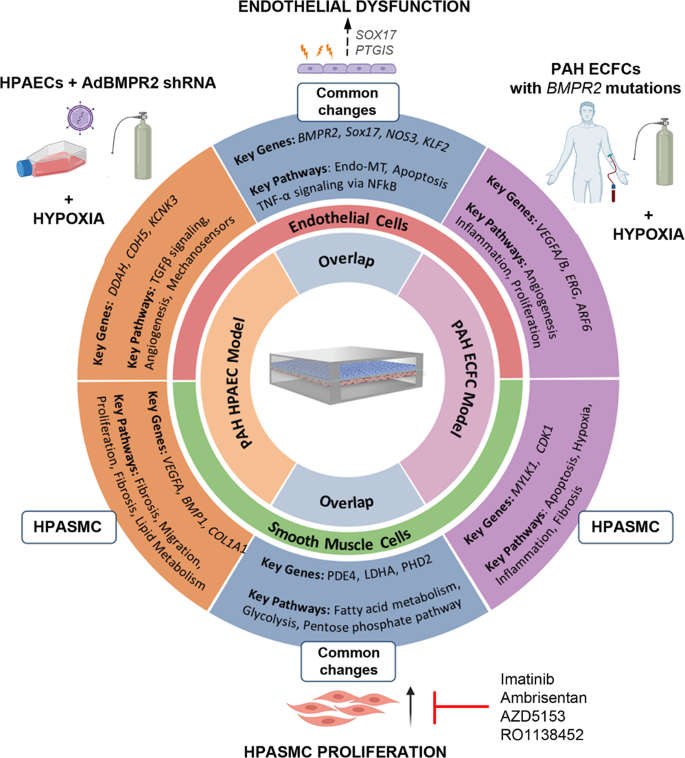
AdBMPR2 shRNA-treated HPAECs and PAH ECFCs with disabling BMPR2 mutations were co-cultured with HPASMCs under hypoxic (2% O2) conditions in PA-on-a-chip for 24 h. The diagram shows selected key PAH genes and pathways identified by transcriptomic profiling of endothelial and smooth muscle cells.
Most BMPR2 mutation carriers and heterozygous BMPR2 knockout animals do not spontaneously develop PAH and a second hit is required for the full manifestation of the disease4. Consistent with this premise, a combination of endothelial BMPR2 knockdown with hypoxia in PA-on-a-chip was required to induce HPASMC proliferation. The mechanisms by which endothelial BMPR2 depletion primes vascular cells for the disease and converges with the effects of the second hit are not well understood. Using a combination of functional assays, RNA sequencing and pathway analysis in human pulmonary endothelial and smooth muscle cells and cells from PAH patients with disabling BMPR2 mutations under the two-hit conditions, we have identified a microfluidic signature of vascular responses characteristic of PAH. Crucially, this gene dataset showed a substantial overlap with transcriptomic changes reported in PAH and identified gene targets of potential interest.
Loss of BMPR2 in HPAECs inhibited expression of TGF-β family genes involved in vascular differentiation, angiogenesis and vessel maturation30, consistent with changes reported in PAH. HPAECs also showed reduced expression of junctional markers CDH5 (VE-cadherin), GJA5 (connexin 40) and several integrins, likely to predispose the endothelium to stress-induced damage.
BMPR2-deficient HPAECs also showed reduced expression of NO bioavailability enzymes, NOS3, DDAH1 and DDAH2, consistent with PAH vasculopathy31,32. Other key PAH-related changes included a reduction in arterial identity factors SOX17, SOX18, and KLF2 as well as regulators of angiogenesis, proliferation, and endothelial sprouting behaviour. Exposure of these BMPR2-deficient endothelial cells to hypoxia altered the expression of genes controlling oxygen transport, cell cycle, transcriptional regulation and inflammation, including genes with well-documented links to PAH: KCNK33,34, HDAC435, BRD412, CAMK2G36,37, and ICAM138. To summarise, the two-hit microfluidic model offers insight into processes seen during the development of the disease, associated with a loss of arterial identity, reduced eNOS signalling and increased susceptibility to damage, accompanied by inflammation and metabolic shift towards glycolysis.
Differential gene expression in HPASMC co-cultured with BMPR2-deficient HPAECs in the two-hit PAH model was enriched for disease-specific phenotypes associated with angiogenesis, apoptosis, inflammation, vasoconstriction and TGF-β signalling. Downregulation of extracellular matrix genes (collagens, fibronectin, laminin, tenascin), modulators of SMC migration and proliferation (serpin, periostin, caveolin39 and apelin40) and upregulation of matrix metalloproteinases is likely to compromise arterial structural integrity and stimulate SMC migration and proliferation41,42. Another notable observation involved a reduction in plasmalemmal potassium channels, KCNMA, KCNAB2, and KCNK1 in these cells, a change linked with pulmonary vasoconstriction and vascular remodelling43. Consistent with the pro-proliferative phenotype of HPASMCs under the double hit conditions, we observed a 6-fold increase in the activity of hexokinase, a key enzyme that controls the rate of glycolysis and pentose cycle, the two key pathways which provide energy and metabolites for the rapidly growing cells.
Patient blood-derived endothelial colony-forming cells (ECFCs) display abnormalities characteristic of PAH, raising interest in their application in personalised medicine20,21. To validate our model, we used patient ECFCs with disabling BMPR2 mutations in place of BMPR2-depleted HPAECs. The comparative analysis of cell responses revealed several important similarities between the two cell types. Consistent with changes seen in BMPR2-deficient HPAECs, ECFCs with BMPR2 mutations showed upregulation of inflammatory genes (IL6, REL, NFKB1, NFKB2, CXCL1, 2, 3, 8, TRAF1, ICAM3), glycolysis (HK2, LDHA, PDK4) and mitochondrial function (FIS1, STOML2)44 and downregulation of key markers of arterial identity (ERG and SOX17)45. However, in contrast with the response seen in HPAECs, ECFCs showed an upregulation of genes regulating NO bioavailability (NOS3, DDAH1, DDAH2) and increased expression of genes promoting endothelial repair and angiogenesis (VEGFA, VEGFB, FGF2, CLIC1, RAC1, ROCK1, ARF6, KLF2, KLF6, KLF7), which may be reflective of the uncontrolled propagation of reparative endothelial cell responses in advanced PAH46.
A link between BMPR2 and SOX17 reported in this study, is intriguing, considering the association of variation in both genes with susceptibility to PAH5,47. The loss of endothelial SOX17 was necessary for the induction of PASMCs proliferation under the two-hit conditions and we identified prostacyclin synthase (PTGIS) and prostacyclin, as likely mediators of this response. Establishing the precise nature of SOX17 interactions with PTGIS and other genes involved in pathological processes in PAH such as PTGR248, SOD149, Wnt pathway modulator, DKK250,51 or lipid metabolism and insulin resistance regulator, KLF1652 is beyond the scope of this study and will require further investigation.
The clinically approved endothelin receptor antagonist, Ambrisentan, and a novel drug candidate, BRD4 inhibitor, AZD5153 were effective in inhibiting PASMC proliferation in the inducible microfluidic models featuring BMPR2-deficient HPAECs and patient-derived ECFCs. BRD4, a member of the BET (bromodomain and extra-terminal motif) family, is a key transcriptional modulator of cell apoptosis and proliferation and a critical epigenetic driver for cardiovascular diseases12. Consistent with the observations in PAH lungs12, expression of BRD4 was significantly elevated in HPAECs cultured under the two-hit conditions, compared with healthy, untreated controls.
The economic use of cells, short experimental time, device scalability, and low production and operation cost make our PA-on-a-chip an attractive model for drug testing. The only other existing PAH-on-a-chip model53,54 included intimal, medial, and adventitial cells from PAH pulmonary arteries grown in straight channels arranged side-by-side, with cell-to-cell contact restricted to the gaps between silicone posts situated between the channels54. This model can provide valuable information regarding paracrine regulation of cell migration but cannot evaluate endothelial barrier function and is limited by poor accessibility of patient lung material, which precludes its wider application in research and drug testing. Our device, which accommodates only two cell types, is unlikely to fully reflect the complex pathophysiology of PAH and therefore future sourcing of patient-derived cells and introducing other cell types will be a vital consideration. The current design should be further improved to allow the migration of cells between the two vascular layers, a response linked to vascular remodelling in PAH. One such approach would be increasing the size of pores in the membrane separating the two microfluidic channels.
Animal models have been instrumental in deciphering the molecular mechanisms underlying the disease but are often criticised for not fully reproducing the pathology of human disease and for low success in the clinical translation of new drugs8. The benefits and limitations of animal models of PAH as well as new measures undertaken to improve their translational value have been summarised in the two excellent recent reviews8,55,56. Our model successfully reproduced the disease characteristics previously described in human and animal PAH, such as medial cell proliferation and activation of PAH-associated pathways in pulmonary arterial intimal and medial cells. However, considering the limitations of this model, such as lack of complexity, variability associated with genetic diversity, age and comorbiditie...
Comments
Post a Comment